Characterization of the structure-function relationship of a novel salt-resistant antimicrobial peptide, RR12
- PMID: 35517355
- PMCID: PMC9054785
- DOI: 10.1039/d0ra04299d
Characterization of the structure-function relationship of a novel salt-resistant antimicrobial peptide, RR12
Abstract
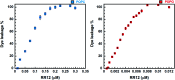
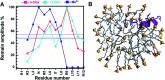
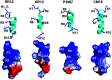
Antimicrobial peptides (AMPs) are potential candidates in designing new anti-infective agents. However, many AMPs show poor bactericidal activities in physical salt and serum solutions. Here, we disclosed the structure-function relationships of a novel salt-resistant antimicrobial peptide, RR12, which could further explain its mode of action and show its applicability in developing new antibacterial agents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Salt-resistant short antimicrobial peptides.Biopolymers. 2016 May;106(3):345-56. doi: 10.1002/bip.22819. Biopolymers. 2016. PMID: 26849911
-
Design of short membrane selective antimicrobial peptides containing tryptophan and arginine residues for improved activity, salt-resistance, and biocompatibility.Biotechnol Bioeng. 2014 Jan;111(1):37-49. doi: 10.1002/bit.25003. Epub 2013 Aug 5. Biotechnol Bioeng. 2014. PMID: 23860860
-
Truncated antimicrobial peptides from marine organisms retain anticancer activity and antibacterial activity against multidrug-resistant Staphylococcus aureus.Peptides. 2013 Jun;44:139-48. doi: 10.1016/j.peptides.2013.04.004. Epub 2013 Apr 15. Peptides. 2013. PMID: 23598079
-
Alpha-helical cationic antimicrobial peptides: relationships of structure and function.Protein Cell. 2010 Feb;1(2):143-52. doi: 10.1007/s13238-010-0004-3. Epub 2010 Feb 6. Protein Cell. 2010. PMID: 21203984 Free PMC article. Review.
-
Dimerization of Antimicrobial Peptides: A Promising Strategy to Enhance Antimicrobial Peptide Activity.Protein Pept Lett. 2019;26(2):98-107. doi: 10.2174/0929866526666190102125304. Protein Pept Lett. 2019. PMID: 30605048 Free PMC article. Review.
Cited by
-
Anti-methicillin-resistant Staphylococcus aureus and antibiofilm activity of new peptides produced by a Brevibacillus strain.PeerJ. 2023 Oct 2;11:e16143. doi: 10.7717/peerj.16143. eCollection 2023. PeerJ. 2023. PMID: 37810790 Free PMC article.
-
Deciphering Structure-Function Relationship Unveils Salt-Resistant Mode of Action of a Potent MRSA-Inhibiting Antimicrobial Peptide, RR14.J Bacteriol. 2022 Dec 20;204(12):e0031222. doi: 10.1128/jb.00312-22. Epub 2022 Nov 15. J Bacteriol. 2022. PMID: 36377870 Free PMC article.
-
Structural and Functional Enrichment Analyses for Antimicrobial Peptides.Int J Mol Sci. 2020 Nov 20;21(22):8783. doi: 10.3390/ijms21228783. Int J Mol Sci. 2020. PMID: 33233636 Free PMC article.
References
LinkOut - more resources
Full Text Sources

