LASS2 impairs proliferation of glioma stem cells and migration and invasion of glioma cells mainly via inhibition of EMT and apoptosis promotion
- PMID: 35517425
- PMCID: PMC9066216
- DOI: 10.7150/jca.71256
LASS2 impairs proliferation of glioma stem cells and migration and invasion of glioma cells mainly via inhibition of EMT and apoptosis promotion
Abstract
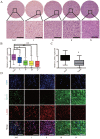
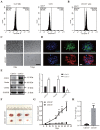
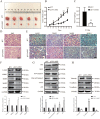
LAG1 longevity assurance homolog 2 (LASS2), a highly conserved transmembrane protein, has been reported in several cancer types. However, the roles of LASS2 in glioma biology remain elusive. In the present study, we investigated the expression of LAAS2 in human glioma tissues and the effects of LASS2 on glioma stem cell (GSC) proliferation. Roles of LASS2 in glioma cell migration and invasion were also researched both in vitro and in vivo. Our results demonstrated that the level of LASS2 is gradually reduced with the increase of glioma grade. The level of LASS2 is significantly lower in GSCs than in non GSCs, whereas LASS2 overexpression reduced the sphere formation and promoted the differentiation of CD133+ glioblastoma cells, as was indicated by reduced levels of CD133 and Nestin. In addition, LASS2 overexpression significantly reduced colony formation, migration, and invasion of glioma cells by promoting tumor cell apoptosis and inhibiting epithelial-mesenchymal transition (EMT). Overexpression of LASS2 inhibited U-87 MG cell-derived glioma xenograft growth in nude mice in a manner similar to in vitro. Our findings indicate that LASS2 can function as a suppressor of glioma growth, suggesting that modulation of LASS2 expression may contribute to a novel strategy for the management of glioma via inhibition of GSCs.
Keywords: LASS2; epithelial-mesenchymal transition (EMT); glioblastoma; glioma; glioma stem cells; invasion; migration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432–46. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. - PubMed
-
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer research. 2004;64:7011–21. - PubMed
-
- Tunici P, Irvin D, Liu G, Yuan X, Zhaohui Z, Ng H. et al. Brain tumor stem cells: new targets for clinical treatments? Neurosurgical focus. 2006;20:E27. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

