Iodine-catalyzed efficient synthesis of xanthene/thioxanthene-indole derivatives under mild conditions
- PMID: 35517437
- PMCID: PMC9055358
- DOI: 10.1039/d0ra05217e
Iodine-catalyzed efficient synthesis of xanthene/thioxanthene-indole derivatives under mild conditions
Abstract
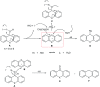
An iodine-catalyzed nucleophilic substitution reaction of xanthen-9-ol and thioxanthen-9-ol with indoles has been developed, providing an efficient procedure for the synthesis of xanthene/thioxanthene-indole derivatives with good to excellent yields. This protocol offers several advantages, such as short reaction times, green solvent, operational simplicity, easily available catalyst and mild reaction conditions. Moreover, this method showed good tolerance of functional groups and a wide range of substrates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Taghartapeh M. R. Pesyan N. N. Rashidnejad H. Khavasi H. R. Soltani A. J. Mol. Struct. 2017;1149:862. doi: 10.1016/j.molstruc.2017.08.054. - DOI
- Jeyaseeli L. Gupta A. D. Kumar K. A. Mazumdar K. Dutta N. K. Dastidar S. G. Int. J. Antimicrob. Agents. 2006;27:58. doi: 10.1016/j.ijantimicag.2005.08.014. - DOI - PubMed
- Yıldız T. Kücük H. B. RSC Adv. 2017;7:16644. doi: 10.1039/C6RA27094H. - DOI
-
- Wu S. Y. Fu Y. H. Chen G. Y. Li X. B. Zhou Q. Han C. R. Song X. P. Du X. J. Xie M. L. Yao G. G. Phytochem. Lett. 2015;11:236. doi: 10.1016/j.phytol.2015.01.002. - DOI
-
- Naseem S. Khalid M. Tahir M. N. Halim M. A. Braga A. A. C. Naseer M. M. Shafia Z. J. Mol. Struct. 2017;1143:235. doi: 10.1016/j.molstruc.2017.04.093. - DOI
- Barmak A. Niknam K. Mohebbi G. Pournabi H. Microb. Pathog. 2019;130:95. doi: 10.1016/j.micpath.2019.03.002. - DOI - PubMed
- Akbari A. Hosseini-Nia A. J. Saudi Chem. Soc. 2017;21:S7. doi: 10.1016/j.jscs.2013.09.009. - DOI
-
- Hafez H. N. Hegab M. I. Ahmed-Farag I. S. El-Gazzar A. B. A. Bioorg. Med. Chem. Lett. 2008;18:4538. doi: 10.1016/j.bmcl.2008.07.042. - DOI - PubMed
- Banerjee A. G. Kothapalli L. P. Sharma P. A. Thomas A. B. Nanda R. K. Shrivastava S. K. Khatanglekar V. V. Arabian J. Chem. 2016;9:S480. doi: 10.1016/j.arabjc.2011.06.001. - DOI
LinkOut - more resources
Full Text Sources