A dual emission metal-organic framework for rapid ratiometric fluorescence detection of CO3 2- in seawater
- PMID: 35517457
- PMCID: PMC9055149
- DOI: 10.1039/d0ra02581j
A dual emission metal-organic framework for rapid ratiometric fluorescence detection of CO3 2- in seawater
Abstract
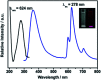
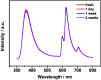
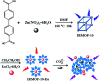
A dual emission metal-organic framework (IRMOF-10-Eu) was prepared and used as a ratiometric fluorescent sensor for CO3 2- detection. IRMOF-10-Eu had good stability and excellent luminescence in aqueous solution. IRMOF-10-Eu showed dual fluorescence emission from the ligand and Eu3+ with single excitation. Upon treatment with CO3 2-, the fluorescence ratio (I 624/I 358) of the probe displayed significant change. The relative fluorescence intensity ratio (I 624/I 358) and CO3 2- concentration had a linear relationship in 50-300 μM range with a low detection limit of 9.58 μM. And the luminescence probe of CO3 2- showed a fast detection time. The possible mechanism was investigated. CO3 2- changed the structure of IRMOF-10-Eu and interrupted the energy transfer process. Thus, the fluorescence emission intensity of the ligand was increased and Eu3+ was decreased with the addition of CO3 2-. IRMOF-10-Eu was used to detect CO3 2- in seawater, which showed good prospect in practical application. Subsequently, a highly selective and sensitive probe, IRMOF-10-Eu, may pave an efficient way for CO3 2- detection in seawater.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures



Similar articles
-
Ratiometric fluorescent sensor based on metal-organic framework for selective and sensitive detection of CO32.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Oct 15;299:122844. doi: 10.1016/j.saa.2023.122844. Epub 2023 May 10. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 37196552
-
A Dual-emitting Two-dimensional Nickel-based Metal-organic Framework Nanosheets: Eu3+/Ag+ Functionalization Synthesis and Ratiometric Sensing in Aqueous Solution.J Fluoresc. 2021 Nov;31(6):1947-1957. doi: 10.1007/s10895-021-02826-w. Epub 2021 Sep 21. J Fluoresc. 2021. PMID: 34546469
-
In-situ synthesis of carbon dots-embedded europium metal-organic frameworks for ratiometric fluorescence detection of Hg2+ in aqueous environment.Anal Chim Acta. 2021 Jan 2;1141:13-20. doi: 10.1016/j.aca.2020.10.028. Epub 2020 Oct 19. Anal Chim Acta. 2021. PMID: 33248646
-
Rational design of dual-ligand Eu-MOF for ratiometric fluorescence sensing Cu2+ ions in human serum to diagnose Wilson's disease.Anal Chim Acta. 2022 Apr 29;1204:339731. doi: 10.1016/j.aca.2022.339731. Epub 2022 Mar 16. Anal Chim Acta. 2022. PMID: 35397914
-
Isoreticular Metal-Organic Framework-3 (IRMOF-3): From Experimental Preparation, Functionalized Modification to Practical Applications.Polymers (Basel). 2024 Jul 26;16(15):2134. doi: 10.3390/polym16152134. Polymers (Basel). 2024. PMID: 39125160 Free PMC article. Review.
Cited by
-
Preparation, Characterization, and In Vitro Release of Curcumin-Loaded IRMOF-10 Nanoparticles and Investigation of Their Pro-Apoptotic Effects on Human Hepatoma HepG2 Cells.Molecules. 2022 Jun 20;27(12):3940. doi: 10.3390/molecules27123940. Molecules. 2022. PMID: 35745062 Free PMC article.
-
Selectivity Enhancement for Uric Acid Detection via In Situ Preparation of Blue Emissive Carbon Dots Entrapped in Chromium Metal-Organic Frameworks.ACS Omega. 2022 May 5;7(19):16576-16583. doi: 10.1021/acsomega.2c00790. eCollection 2022 May 17. ACS Omega. 2022. PMID: 35601314 Free PMC article.
References
-
- Tir N. Al Ezzi M. M. Abdullah H. M. Yusupov M. Kouser S. Bahlouli H. Yamani Z. H. Detection of CO2 using CNT-based sensors: role of Fe catalyst on sensitivity and selectivity. Mater. Chem. Phys. 2017;186:353–364. doi: 10.1016/j.matchemphys.2016.11.006. - DOI
-
- Feely R. A. Alin S. R. Newton J. Sabine C. L. Warner M. Devol A. Krembs C. Maloy C. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast Shelf Sci. 2010;88:441–449. doi: 10.1016/j.ecss.2010.05.004. - DOI
-
- He L. Liu C. Xin J. H. A novel turn-on colorimetric and fluorescent sensor for Fe3+ and Al3+ with solvent-dependent binding properties and its sequential response to carbonate. Sens. Actuators, B. 2015;213:181–187. doi: 10.1016/j.snb.2015.02.060. - DOI
-
- Ghorai A. Mondal J. Chandra R. Patra G. K. A reversible fluorescent-colorimetric chemosensor based on a novel Schiff base for visual detection of CO32− in aqueous solution. RSC Adv. 2016;6:72185–72192. doi: 10.1039/C5RA24549D. - DOI
LinkOut - more resources
Full Text Sources

