Synthesis of 2,5-diaryloxazoles through rhodium-catalyzed annulation of triazoles and aldehydes
- PMID: 35517461
- PMCID: PMC9055139
- DOI: 10.1039/d0ra03966g
Synthesis of 2,5-diaryloxazoles through rhodium-catalyzed annulation of triazoles and aldehydes
Abstract
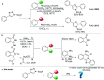
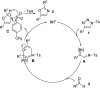
An efficient synthesis of a variety of 2,5-diaryloxazole derivatives via a rhodium-catalyzed annulation of triazoles and aldehydes is achieved. Various oxazole derivatives could be obtained in good to excellent yields. A concise synthesis of antimycobaterial natural products balsoxin and texamine has been achieved using this method.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Wipf P. Chem. Rev. 1995;95:2115–2134. doi: 10.1021/cr00038a013. - DOI
- Oxazoles: Synthesis, Reactions and Spectroscopy, Part B, ed. D. C. Palmer, John Wiley & Sons, Hoboken, NJ, 2004
- Nicolaou K. C. Hao J. Reddy M. V. Rao P. B. Rassias G. Snyder S. A. Huang X. Chen D. Y. K. Brenzovich W. E. Giuseppone N. Giannakakou P. O'Brate A. J. Am. Chem. Soc. 2004;126:12897. doi: 10.1021/ja040093a. - DOI - PubMed
- Jin Z. Nat. Prod. Rep. 2011;28:1143. doi: 10.1039/C0NP00074D. - DOI - PubMed
- Zhang J. Ciufolini M. A. Org. Lett. 2011;13:390. doi: 10.1021/ol102678j. - DOI - PubMed
- Imai S. Kikui H. Moriyama K. Togo H. Tetrahedron. 2015;71:5267. doi: 10.1016/j.tet.2015.06.022. - DOI
- Chiacchio M. A. Lanza G. Chiacchio U. Giofre S. V. Romeo R. Iannazzo D. Legnani L. Curr. Med. Chem. 2019;41:7337. - PubMed
-
- Nagatsu A. Kajitani H. Sakakibara J. Tetrahedron Lett. 1995;36:4097. doi: 10.1016/0040-4039(95)00724-Q. - DOI
- Mattila A. Andsten R. M. Jumppanen M. Assante M. Jokela J. Wahlsten M. Mikula K. M. Sigindere C. Kwak D. H. Gugger M. Koskela H. Sivonen K. Liu X. Y. Yli-Kauhaluoma J. Iwai H. Fewer D. P. ACS Chem. Biol. 2019;12:2683. doi: 10.1021/acschembio.9b00620. - DOI - PubMed
LinkOut - more resources
Full Text Sources