Copper(ii)-catalyzed synthesis of multisubstituted indoles through sequential Chan-Lam and cross-dehydrogenative coupling reactions
- PMID: 35517475
- PMCID: PMC9055228
- DOI: 10.1039/d0ra04592f
Copper(ii)-catalyzed synthesis of multisubstituted indoles through sequential Chan-Lam and cross-dehydrogenative coupling reactions
Abstract
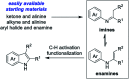
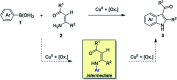
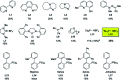
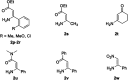
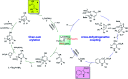
Starting from arylboronic acids and ester (Z)-3-aminoacrylates, one-pot syntheses of diverse indole-3-carboxylic esters have been described through copper(ii)-catalyzed sequential Chan-Lam N-arylation and cross-dehydrogenative coupling (CDC) reactions. The initial Chan-Lam arylation can proceed in DMF at 100 °C for 24 h to give ester (Z)-3-(arylamino)acrylate intermediates in the presence of Cu(OAc)2/tri-tert-butylphosphine tetrafluoroborate, a catalytic amount of myristic acid as the additive, KMnO4 and KHCO3. Sequentially, these in situ arylated intermediates can undergo an intramolecular oxidative cross-dehydrogenative coupling process in mixed solvents (DMF/DMSO = 2 : 1) at 130 °C to give C3-functionalized multi-substituted indole derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Autocatalytic photoredox Chan-Lam coupling of free diaryl sulfoximines with arylboronic acids.Nat Commun. 2021 Feb 10;12(1):932. doi: 10.1038/s41467-021-21156-w. Nat Commun. 2021. PMID: 33568641 Free PMC article.
-
Copper-Catalyzed Synthesis of Multisubstituted Indoles through Tandem Ullmann-Type C-N Formation and Cross-dehydrogenative Coupling Reactions.J Org Chem. 2018 May 4;83(9):5288-5294. doi: 10.1021/acs.joc.8b00353. Epub 2018 Apr 19. J Org Chem. 2018. PMID: 29664297
-
Copper(ii)-catalyzed Chan-Lam cross-coupling: chemoselective N-arylation of aminophenols.Org Biomol Chem. 2017 Jan 25;15(4):801-806. doi: 10.1039/c6ob02444k. Org Biomol Chem. 2017. PMID: 28045171
-
Recent Developments in Arylation of N-Nucleophiles via Chan-Lam Reaction: Updates from 2012 Onwards.Curr Org Synth. 2022;19(1):16-30. doi: 10.2174/1570179417666210105120706. Curr Org Synth. 2022. PMID: 33402088 Review.
-
Recent Progress Concerning the N-Arylation of Indoles.Molecules. 2021 Aug 22;26(16):5079. doi: 10.3390/molecules26165079. Molecules. 2021. PMID: 34443667 Free PMC article. Review.
Cited by
-
CuI-catalyzed synthesis of multisubstituted pyrido[1,2-a]pyrimidin-4-ones through tandem Ullmann-type C-N cross-coupling and intramolecular amidation reaction.RSC Adv. 2023 Aug 14;13(35):24264-24271. doi: 10.1039/d3ra04454h. eCollection 2023 Aug 11. RSC Adv. 2023. PMID: 37583662 Free PMC article.
References
-
- de Sa Alves F. R. Barreiro E. J. Fraga C. A. M. Mini-Rev. Med. Chem. 2009;9:782. doi: 10.2174/138955709788452649. - DOI - PubMed
- Sravanthi T. V. Manju S. L. Eur. J. Pharm. Sci. 2016;91:1. doi: 10.1016/j.ejps.2016.05.025. - DOI - PubMed
- Kaushik N. K. Kaushik N. Attri P. Kumar N. Kim C. H. Verma A. K. Choi E. H. Molecules. 2013;18:6620. doi: 10.3390/molecules18066620. - DOI - PMC - PubMed
- Singh T. P. Singh O. M. Mini-Rev. Med. Chem. 2018;18:9. doi: 10.2174/1389557517666170807124507. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

