Inflammasomes in Cancer Progression and Anti-Tumor Immunity
- PMID: 35517498
- PMCID: PMC9065266
- DOI: 10.3389/fcell.2022.839041
Inflammasomes in Cancer Progression and Anti-Tumor Immunity
Abstract
The inflammasomes are critical regulators of innate immunity, inflammation and cell death and have emerged as important regulators of cancer development and control. Inflammasomes are assembled by pattern recognition receptors (PRR) following the sensing of microbial- or danger-associated molecular patterns (MAMPs/DAMPs) and elicit inflammation through the oligomerization and activation of inflammatory caspases. These cysteinyl-aspartate proteases cleave the proinflammatory cytokines IL-1β and IL-18 into their biologically active mature form. The roles of the inflammasomes and associated pro-inflammatory cytokines vary greatly depending on the cancer type. Here we discuss recent studies highlighting contrasting roles of the inflammasome pathway in curbing versus promoting tumorigenesis. On one hand, the inflammasomes participate in stimulating anti-tumor immunity, but they have also been shown to contribute to immunosuppression or to directly promote tumor cell survival, proliferation, and metastasis. A better understanding of inflammasome functions in different cancers is thus critical for the design of novel cancer immunotherapies.
Keywords: Inflammasome; caspase; immunosuppres-sion; immunotherapy; macrophages; metastasis; pyroptosis; tumor.
Copyright © 2022 Lillo and Saleh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
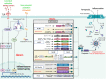

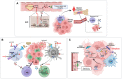
Similar articles
-
Targeting inflammasomes as an immunotherapeutic strategy for cancer.J Transl Med. 2025 Jun 8;23(1):634. doi: 10.1186/s12967-025-06665-2. J Transl Med. 2025. PMID: 40484954 Free PMC article. Review.
-
The inflammasomes: molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections.Front Microbiol. 2011 Feb 17;2:15. doi: 10.3389/fmicb.2011.00015. eCollection 2011. Front Microbiol. 2011. PMID: 21716947 Free PMC article.
-
Inflammasome as a New Therapeutic Target for Diabetic Complications.Recent Pat Endocr Metab Immune Drug Discov. 2016;10(1):56-62. doi: 10.2174/1872214810666160219163314. Recent Pat Endocr Metab Immune Drug Discov. 2016. PMID: 26899852 Review.
-
Pyroptotic and non-pyroptotic effector functions of caspase-11.Immunol Rev. 2020 Sep;297(1):39-52. doi: 10.1111/imr.12910. Epub 2020 Aug 1. Immunol Rev. 2020. PMID: 32737894 Free PMC article. Review.
-
[The inflammasomes: platforms of innate immunity].Med Sci (Paris). 2013 Nov;29(11):975-84. doi: 10.1051/medsci/20132911013. Epub 2013 Nov 20. Med Sci (Paris). 2013. PMID: 24280500 Review. French.
Cited by
-
Transcriptomic analysis of glucosidase II beta subunit (GluIIß) knockout A549 cells reveals its roles in regulation of cell adhesion molecules (CAMs) and anti-tumor immunity.BMC Genomics. 2024 Jan 20;25(1):82. doi: 10.1186/s12864-023-09888-z. BMC Genomics. 2024. PMID: 38245670 Free PMC article.
-
Cell death, therapeutics, and the immune response in cancer.Trends Cancer. 2023 May;9(5):381-396. doi: 10.1016/j.trecan.2023.02.001. Epub 2023 Feb 24. Trends Cancer. 2023. PMID: 36841748 Free PMC article. Review.
-
Inflammasomes in lymphocytes as therapeutic targets.Transl Oncol. 2025 Apr;54:102342. doi: 10.1016/j.tranon.2025.102342. Epub 2025 Mar 6. Transl Oncol. 2025. PMID: 40054124 Free PMC article.
-
Cholesterol crystals in the pathogenesis of atherosclerosis.Nat Rev Cardiol. 2025 May;22(5):315-332. doi: 10.1038/s41569-024-01100-3. Epub 2024 Nov 18. Nat Rev Cardiol. 2025. PMID: 39558130 Review.
-
Targeting inflammasomes as an immunotherapeutic strategy for cancer.J Transl Med. 2025 Jun 8;23(1):634. doi: 10.1186/s12967-025-06665-2. J Transl Med. 2025. PMID: 40484954 Free PMC article. Review.
References
-
- Brunetto E., De Monte L., Balzano G., Camisa B., Laino V., Riba M., et al. (2019). The IL-1/IL-1 Receptor axis and Tumor Cell Released Inflammasome Adaptor ASC Are Key Regulators of TSLP Secretion by Cancer Associated Fibroblasts in Pancreatic Cancer. J. Immunotherapy Cancer 7, 45. 10.1186/s40425-019-0521-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

