Canonical Wnt Signaling Promotes Formation of Somatic Permeability Barrier for Proper Germ Cell Differentiation
- PMID: 35517512
- PMCID: PMC9062081
- DOI: 10.3389/fcell.2022.877047
Canonical Wnt Signaling Promotes Formation of Somatic Permeability Barrier for Proper Germ Cell Differentiation
Abstract
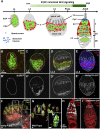
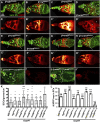
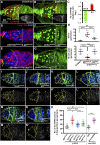
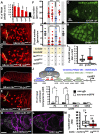
Morphogen-mediated signaling is critical for proper organ development and stem cell function, and well-characterized mechanisms spatiotemporally limit the expression of ligands, receptors, and ligand-binding cell-surface glypicans. Here, we show that in the developing Drosophila ovary, canonical Wnt signaling promotes the formation of somatic escort cells (ECs) and their protrusions, which establish a physical permeability barrier to define morphogen territories for proper germ cell differentiation. The protrusions shield germ cells from Dpp and Wingless morphogens produced by the germline stem cell (GSC) niche and normally only received by GSCs. Genetic disruption of EC protrusions allows GSC progeny to also receive Dpp and Wingless, which subsequently disrupt germ cell differentiation. Our results reveal a role for canonical Wnt signaling in specifying the ovarian somatic cells necessary for germ cell differentiation. Additionally, we demonstrate the morphogen-limiting function of this physical permeability barrier, which may be a common mechanism in other organs across species.
Keywords: DPP; GSCs; TKV; Wg; escort cell protrusions; germline stem cells.
Copyright © 2022 Chen, Lin, Yang, Tseng, Wang, Lin, Luo, Cai and Hsu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A switch in the mode of Wnt signaling orchestrates the formation of germline stem cell differentiation niche in Drosophila.PLoS Genet. 2018 Jan 25;14(1):e1007154. doi: 10.1371/journal.pgen.1007154. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29370168 Free PMC article.
-
Smad-Independent BMP Signaling in Somatic Cells Limits the Size of the Germline Stem Cell Pool.Stem Cell Reports. 2018 Sep 11;11(3):811-827. doi: 10.1016/j.stemcr.2018.07.008. Epub 2018 Aug 16. Stem Cell Reports. 2018. PMID: 30122445 Free PMC article.
-
Traffic jam regulates the function of the ovarian germline stem cell progeny differentiation niche during pre-adult stage in Drosophila.Sci Rep. 2019 Jul 12;9(1):10124. doi: 10.1038/s41598-019-45317-6. Sci Rep. 2019. PMID: 31300663 Free PMC article.
-
Wnt Signaling in Stem Cell Maintenance and Differentiation in the Drosophila Germarium.Genes (Basel). 2018 Feb 27;9(3):127. doi: 10.3390/genes9030127. Genes (Basel). 2018. PMID: 29495453 Free PMC article. Review.
-
Role of Chromatin Modifications in Drosophila Germline Stem Cell Differentiation.Results Probl Cell Differ. 2017;59:1-30. doi: 10.1007/978-3-319-44820-6_1. Results Probl Cell Differ. 2017. PMID: 28247044 Review.
Cited by
-
Steroid hormone-induced wingless ligands tune female intestinal size in Drosophila.Nat Commun. 2025 Jan 6;16(1):436. doi: 10.1038/s41467-024-55664-2. Nat Commun. 2025. PMID: 39762218 Free PMC article.
References
-
- Ashburner M. (2005). Drosophila: A Laboratory Handbook. NY: Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

