Synthesis of aryl azide chain-end functionalized N-linked glycan polymers and their photo-labelling of specific protein
- PMID: 35517525
- PMCID: PMC9057295
- DOI: 10.1039/d0ra08400j
Synthesis of aryl azide chain-end functionalized N-linked glycan polymers and their photo-labelling of specific protein
Abstract
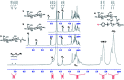
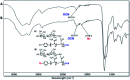
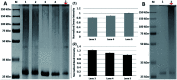
We report a straightforward synthesis of aryl azide chain-end functionalized N-linked glycan polymers and its application for affinity-assisted photo-labelling of specific protein. The aryl azide chain-end functionalized N-glycan polymers, including N-galactosyl, N-glucosyl, and N-lactosyl polymer, were synthesized from free glycan via glycosylamine intermediates followed by acrylation and polymerization via cyanoxyl-mediated free radical polymerization (CMFRP) in a one-pot fashion. The aryl azide chain-end functionalized N-glycan polymers were characterized by 1H NMR and IR spectroscopy. The affinity-assisted photo-labeling capabilities of the aryl azide N-glycan polymers were demonstrated with aryl azide N-lactosyl polymer as a ligand for β-galactose-specific lectin from Arachis hypogaea (PNA) after UV irradiation and confirmed by SDS-PAGE with silver staining. Overall, the aryl azide chain-end functionalized N-linked glycan polymers will be useful multivalent ligands for specific protein labelling and functionality studies.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Synthesis and Characterization of α,ω-End Orthogonally Functionalizable Glycopolymers from Native Glycans.Polym Chem. 2024 May 14;15(18):1884-1896. doi: 10.1039/d4py00191e. Epub 2024 Apr 11. Polym Chem. 2024. PMID: 39679285 Free PMC article.
-
Straightforward Synthesis of N-Glycan Polymers from Free Glycans via Cyanoxyl Free Radical-Mediated Polymerization.ACS Macro Lett. 2017 Feb 21;6(2):107-111. doi: 10.1021/acsmacrolett.6b00928. Epub 2017 Jan 18. ACS Macro Lett. 2017. PMID: 35632901
-
Orientated glyco-macroligand formation based on site-specific immobilization of O-cyanate chain-end functionalized glycopolymer.Org Biomol Chem. 2011 Feb 7;9(3):845-50. doi: 10.1039/c0ob00556h. Epub 2010 Nov 29. Org Biomol Chem. 2011. PMID: 21116566
-
Synthesis of Chain-End Functionalized Glycopolymers via Cyanoxyl-Mediated Free Radical Polymerization (CMFRP).Methods Mol Biol. 2016;1367:3-12. doi: 10.1007/978-1-4939-3130-9_1. Methods Mol Biol. 2016. PMID: 26537460
-
Solvent-Selective Reactions of Alkyl Iodide with Sodium Azide for Radical Generation and Azide Substitution and Their Application to One-Pot Synthesis of Chain-End-Functionalized Polymers.J Am Chem Soc. 2017 Aug 2;139(30):10551-10560. doi: 10.1021/jacs.7b05879. Epub 2017 Jul 25. J Am Chem Soc. 2017. PMID: 28741356
Cited by
-
Synthesis and Characterization of α,ω-End Orthogonally Functionalizable Glycopolymers from Native Glycans.Polym Chem. 2024 May 14;15(18):1884-1896. doi: 10.1039/d4py00191e. Epub 2024 Apr 11. Polym Chem. 2024. PMID: 39679285 Free PMC article.
-
Glycopolymer-Wrapped Carbon Nanotubes Show Distinct Interaction of Carbohydrates With Lectins.Front Chem. 2022 Mar 3;10:852988. doi: 10.3389/fchem.2022.852988. eCollection 2022. Front Chem. 2022. PMID: 35308788 Free PMC article.
References
-
- Fujita A. Kohler J. J. Trends Glycosci. Glycotechnol. 2015;156:E1–E7.
LinkOut - more resources
Full Text Sources

