Probing the supramolecular features via π-π interaction of a di-iminopyrene-di-benzo-18-crown-6-ether compound: experimental and theoretical study
- PMID: 35517536
- PMCID: PMC9057302
- DOI: 10.1039/d0ra06929a
Probing the supramolecular features via π-π interaction of a di-iminopyrene-di-benzo-18-crown-6-ether compound: experimental and theoretical study
Abstract
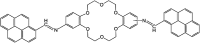
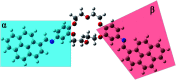
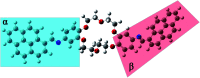
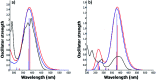
A novel DPyDB-C[double bond, length as m-dash]N-18C6 compound was synthesised by linking a pyrene moiety to each phenyl group of dibenzo-18-crown-6-ether, the crown ether, through -HC[double bond, length as m-dash]N- bonds and characterized by FTIR, 1H-NMR, 13C-NMR, TGA, and DSC techniques. The quantitative 13C-NMR analysis revealed the presence of two position isomers. The electronic structure of the DPyDB-C[double bond, length as m-dash]N-18C6 molecule was characterized by UV-vis and fluorescence spectroscopies in four solvents with different polarities to observe particular behavior of isomers, as well as to demonstrate a possible non-bonding chemical association (such as ground- and excited-state associations, namely, to probe if there were forming dimers/excimers). The interpretation of the electronic structure was realized through QM calculations. The TD-CAM-B3LYP functional, at the 6-311+G(d,p) basis set, indicated the presence of predominant π → π* and mixed π → π* + n → π* transitions, in line with the UV-vis experimental data. Even though DPyDB-C[double bond, length as m-dash]N-18C6 computational studies revealed a π-extended conjugation effect with predominantly π → π* transitions, thorough fluorescence analysis was observed a weak emission, as an effect of PET and ACQ. In particular, the WAXD analysis of powder and thin films obtained from n-hexane, 1,2-dichloroethane, and ethanol indicated an amorphous organization, whereas from toluene a smectic ordering was obtained. These results were correlated with MD simulation, and it was observed that the molecular geometry of DPyDB-C[double bond, length as m-dash]N-18C6 molecule played a defining role in the pyrene stacking arrangement.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Radical anion and dianion salts of titanyl macrocycles with acceptor substituents or an extended π-system.Dalton Trans. 2017 Mar 14;46(11):3547-3555. doi: 10.1039/c6dt04896j. Dalton Trans. 2017. PMID: 28240750
-
Electronic communication in phosphine substituted bridged dirhenium complexes - clarifying ambiguities raised by the redox non-innocence of the C4H2- and C4-bridges.Dalton Trans. 2016 Apr 7;45(13):5783-99. doi: 10.1039/c5dt04768d. Epub 2016 Mar 3. Dalton Trans. 2016. PMID: 26936132
-
Synthesis, structure, and reactivity of iridium perfluorocarbene complexes: regio- and stereo-specific addition of HCl across a metal carbon double bond.Dalton Trans. 2015 Dec 7;44(45):19528-42. doi: 10.1039/c5dt02275d. Epub 2015 Jul 27. Dalton Trans. 2015. PMID: 26211437
-
Syntheses, structures, and physicochemical properties of diruthenium compounds of tetrachlorocatecholate with metal-metal bonded Ru(3+)(mu-OR)(2)Ru(3+) and Ru(3.5+)(mu-OR)(2)Ru(3.5+) cores (R = CH(3) and C(2)H(5)).Inorg Chem. 2001 Jul 2;40(14):3544-54. doi: 10.1021/ic0010252. Inorg Chem. 2001. PMID: 11421704
-
Equilibria and mesomerism/valence tautomerism of group 4 metallocene complexes.Chem Soc Rev. 2020 Apr 7;49(7):2119-2139. doi: 10.1039/c9cs00637k. Chem Soc Rev. 2020. PMID: 32186300 Review.
References
-
- Steed J. W., Atwood J. L. and Gale P. A., in Supramolecular Chemistry: From Molecules to Nanomaterials, 2012
-
- González-Rodríguez D. Schenning A. P. H. J. Chem. Mater. 2011;23:310–325. doi: 10.1021/cm101817h. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

