Synthesis, study of antileishmanial and antitrypanosomal activity of imidazo pyridine fused triazole analogues
- PMID: 35517538
- PMCID: PMC9057266
- DOI: 10.1039/d0ra07881f
Synthesis, study of antileishmanial and antitrypanosomal activity of imidazo pyridine fused triazole analogues
Abstract
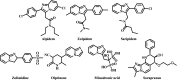
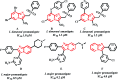
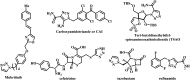
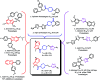
Four groups, thirty-five compounds in total, of novel 1,2,3-triazole analogues of imidazo-[1,2-a]-pyridine-3-carboxamides were designed and synthesized using substituted pyridine, propargyl bromide, 2-azidoethyl 4-methyl benzenesulfonate and substituted acetylenes. These compounds were characterized using 1H NMR, 13C NMR, LCMS and elemental analyses and a crystal structure was obtained for one of the significantly active compounds, 8f. All the synthesized and characterized compounds were screened in vitro for antileishmanial and antitrypanosomal activity against Leishmania major and Trypanosoma brucei parasites, respectively. Among the tested analogues, five compounds (8d, 8f, 8j, 10b and 10d) exhibited significant antileishmanial activity while three compounds (10b, 11a and 11b) showed substantial activity against T. brucei parasite. In silico ADME prediction studies depicted that the essential compounds obeyed Lipinski's rule of five. The predicted in silico toxicity profile suggested that the tested compounds would be non-toxic, which was confirmed experimentally by the lack of cytotoxicity against HeLa cells. Finally, a molecular docking study was also performed, for 10d the most active antileishmanial compound, to study its putative binding pattern at the active site of the selected leishmanial trypanothione reductase target.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare that there is no conflict of interest.
Figures





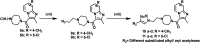


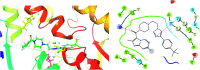

Similar articles
-
Novel phenanthridine amide analogs as potential anti-leishmanial agents: In vitro and in silico insights.Bioorg Chem. 2021 Dec;117:105414. doi: 10.1016/j.bioorg.2021.105414. Epub 2021 Oct 9. Bioorg Chem. 2021. PMID: 34655843
-
Design, synthesis and biological evaluation of novel 1,2,3-triazole analogues of Imidazo-[1,2-a]-pyridine-3-carboxamide against Mycobacterium tuberculosis.Toxicol In Vitro. 2021 Aug;74:105137. doi: 10.1016/j.tiv.2021.105137. Epub 2021 Mar 6. Toxicol In Vitro. 2021. PMID: 33684466
-
Design, synthesis and anti-mycobacterial evaluation of imidazo[1,2-a]pyridine analogues.RSC Med Chem. 2022 Jan 3;13(3):327-342. doi: 10.1039/d1md00367d. eCollection 2022 Mar 23. RSC Med Chem. 2022. PMID: 35434623 Free PMC article.
-
Trypanothione as a target in the design of antitrypanosomal and antileishmanial agents.Curr Pharm Des. 2001 Aug;7(12):1117-41. doi: 10.2174/1381612013397564. Curr Pharm Des. 2001. PMID: 11472257 Review.
-
The Role of Nitro (NO2-), Chloro (Cl), and Fluoro (F) Substitution in the Design of Antileishmanial and Antichagasic Compounds.Curr Drug Targets. 2021;22(4):379-398. doi: 10.2174/1389450121666201228122239. Curr Drug Targets. 2021. PMID: 33371845 Review.
Cited by
-
Novel isatin-triazole based thiosemicarbazones as potential anticancer agents: synthesis, DFT and molecular docking studies.RSC Adv. 2024 Apr 29;14(20):14051-14067. doi: 10.1039/d4ra01937g. eCollection 2024 Apr 25. RSC Adv. 2024. PMID: 38686286 Free PMC article.
-
Synthesis, biological assessment, and computational investigations of nifedipine and monastrol analogues as anti-leishmanial major and anti-microbial agents.Mol Divers. 2023 Dec;27(6):2555-2575. doi: 10.1007/s11030-022-10569-4. Epub 2022 Nov 22. Mol Divers. 2023. PMID: 36417095
-
The Synthesis of Triazolium Salts as Antifungal Agents: A Biological and In Silico Evaluation.Antibiotics (Basel). 2022 Apr 27;11(5):588. doi: 10.3390/antibiotics11050588. Antibiotics (Basel). 2022. PMID: 35625232 Free PMC article.
-
Synthesis of Nitrostyrylthiazolidine-2,4-dione Derivatives Displaying Antileishmanial Potential.Pharmaceuticals (Basel). 2024 Jul 3;17(7):878. doi: 10.3390/ph17070878. Pharmaceuticals (Basel). 2024. PMID: 39065730 Free PMC article.
References
-
- Hotez P. J. and Lo N. C., Neglected Tropical Diseases: Public Health Control Programs and Mass Drug Administration, Elsevier Inc., 10th edn, 2020
LinkOut - more resources
Full Text Sources

