Mesoporous TiO2 anatase films for enhanced photocatalytic activity under UV and visible light
- PMID: 35517541
- PMCID: PMC9057303
- DOI: 10.1039/d0ra06455f
Mesoporous TiO2 anatase films for enhanced photocatalytic activity under UV and visible light
Abstract
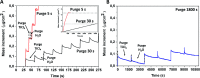
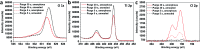
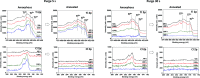
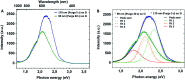
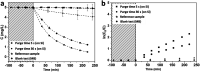
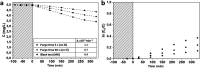
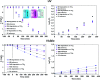
Mesoporous TiO2 films with enhanced photocatalytic activity in both UV and visible wavelength ranges were developed through a non-conventional atomic layer deposition (ALD) process at room temperature. Deposition at such a low temperature promotes the accumulation of by-products in the amorphous TiO2 films, caused by the incomplete hydrolysis of the TiCl4 precursor. The additional thermal annealing induces the fast recrystallisation of amorphous films, as well as an in situ acidic treatment of TiO2. The interplay between the deposition parameters, such as purge time, the amount of structural defects introduced and the enhancement of the photocatalytic properties from different mesoporous films clearly shows that our easily upscalable non-conventional ALD process is of great industrial interest for environmental remediation and other photocatalytic applications, such as hydrogen production.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



References
-
- Hoffmann M. R. Martin S. T. Choi W. Bahnemannt D. W. Chem. Rev. 1995;95:69–96. doi: 10.1021/cr00033a004. - DOI
-
- Ishchenko O. M., Rogé V., Lamblin G. and Lenoble D., in Semiconductor Photocatalysis - Materials, Mechanisms and Applications, ed. W. Cao, IntechOpen, 2016, pp. 3–30
LinkOut - more resources
Full Text Sources

