Effective methods for the synthesis of hydrazones, quinazolines, and Schiff bases: reaction monitoring using a chemometric approach
- PMID: 35517547
- PMCID: PMC9057299
- DOI: 10.1039/d0ra06845d
Effective methods for the synthesis of hydrazones, quinazolines, and Schiff bases: reaction monitoring using a chemometric approach
Abstract
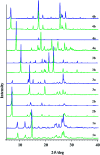
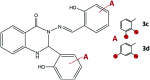
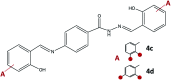
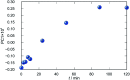
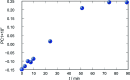
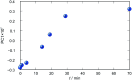
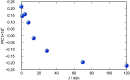
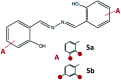
Synthesis of hydrazones (1a-4a and 1b-4b), quinazolines (3c·MeOH and 3d·MeOH), and hydrazone-Schiff bases (4c and 4d) is achieved by combining suitable aldehydes (2,3- or 2,4-dihydroxybenzaldehyde) with four hydrazides (isonicotinic, nicotinic, and 2- or 4-aminobenzoic acid hydrazide). A suite of approaches for their preparation is described: solution-based synthesis, mechanosynthesis, and solid-state melt reactions. The mechanochemical approach is generally a better choice for the quinazolines, while the solid-state melt reaction is more efficient for derivatives of (iso)nicotinic based hydrazones. Crystalline amine-functionalised hydrazones 4a and 4b undergo post-synthetic modifications in reactions with 3- or 4-pyridinecarbaldehyde vapours to form hydrazone-Schiff bases 4a-3py, 4b-3py, 4a-4py, and 4b-4py. Mechanochemical and vapour-mediated reactions are followed by ex situ powder X-ray diffraction and IR-ATR methods, respectively. The chemometric analysis of these data using principal component analysis provided an insight into the reaction profiles and reaction times. Azines (5a and 5b), achieved from aldehydes and hydrazine, reversibly change colour in response to temperature changes. The structures of all products are ascertained by a combined use of spectroscopic and X-ray diffraction methods. The cytotoxic and antimicrobial activities of all compounds against selected human cancer cell lines and bacterial strains are evaluated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




Similar articles
-
Synthesis, admetSAR Predictions, DPPH Radical Scavenging Activity, and Potent Anti-mycobacterial Studies of Hydrazones of Substituted 4-(anilino methyl) benzohydrazides (Part 2).Curr Comput Aided Drug Des. 2021;17(4):493-503. doi: 10.2174/1573409916666200615141047. Curr Comput Aided Drug Des. 2021. PMID: 32538732
-
Synthesis of Isatin Derivatives Exhibiting Antibacterial, Antifungal and Cytotoxic Activities.Curr Org Synth. 2022 Aug 6;19(6):748-756. doi: 10.2174/1570179419666220128091313. Curr Org Synth. 2022. PMID: 35088673
-
New Hydrazides and Hydrazide-Hydrazones of 2,3-Dihalogen Substituted Propionic Acids: Synthesis and in vitro Antimicrobial Activity Evaluation.Chem Biodivers. 2017 Aug;14(8). doi: 10.1002/cbdv.201700075. Epub 2017 Jun 26. Chem Biodivers. 2017. PMID: 28444991
-
Different Schiff Bases-Structure, Importance and Classification.Molecules. 2022 Jan 25;27(3):787. doi: 10.3390/molecules27030787. Molecules. 2022. PMID: 35164049 Free PMC article. Review.
-
An Insight into Fluorinated Imines and Hydrazones as Antibacterial Agents.Int J Mol Sci. 2024 Mar 15;25(6):3341. doi: 10.3390/ijms25063341. Int J Mol Sci. 2024. PMID: 38542315 Free PMC article. Review.
Cited by
-
An Electrosynthesis of 1,3,4-Oxadiazoles from N-Acyl Hydrazones.Chemistry. 2024 Dec 10;30(69):e202403128. doi: 10.1002/chem.202403128. Epub 2024 Oct 30. Chemistry. 2024. PMID: 39291449 Free PMC article.
-
Synthesis, characterization, DFT, antioxidant, antibacterial, pharmacokinetics and inhibition of SARS-CoV-2 main protease of some heterocyclic hydrazones.J Mol Struct. 2022 Dec 15;1270:134005. doi: 10.1016/j.molstruc.2022.134005. Epub 2022 Aug 23. J Mol Struct. 2022. PMID: 36033106 Free PMC article.
-
Synthesis and characterization of indole-3-butyric acid-based hydrazones: from multifunctional enzyme inhibition to therapeutic potential for drug development.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug 18. doi: 10.1007/s00210-025-04461-9. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40820061
-
Synthesis, Structural Properties and Biological Activities of Novel Hydrazones of 2-, 3-, 4-Iodobenzoic Acid.Molecules. 2024 Aug 11;29(16):3814. doi: 10.3390/molecules29163814. Molecules. 2024. PMID: 39202893 Free PMC article.
-
Effect of Structural Variation on Spectral, NLO Properties, and Biological Activity of Pyrrole Hydrazones.ACS Omega. 2022 Aug 16;7(34):29571-29586. doi: 10.1021/acsomega.2c00951. eCollection 2022 Aug 30. ACS Omega. 2022. PMID: 36061655 Free PMC article.
References
-
- Machakanur S. S. Patil B. R. Badiger D. S. Bakale R. P. Gudasi K. B. Bligh S. W. A. J. Mol. Struct. 2012;1011:121–127. doi: 10.1016/j.molstruc.2011.12.023. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

