Stereoselective synthesis and application of isopulegol-based bi- and trifunctional chiral compounds
- PMID: 35517552
- PMCID: PMC9057261
- DOI: 10.1039/d0ra07739a
Stereoselective synthesis and application of isopulegol-based bi- and trifunctional chiral compounds
Abstract
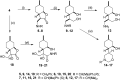
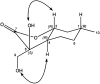
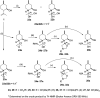
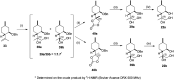
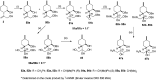
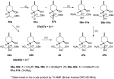
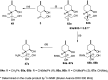
A new family of isopulegol-based bi- and trifunctional chiral ligands was developed from commercially available (-)-isopulegol. Nucleophilic addition of primary amines towards (+)-α-methylene-γ-butyrolactone was accomplished, followed by reduction of the obtained β-aminolactones to provide aminodiols in highly stereoselective reactions. Epoxidation of (-)-isopulegol and subsequent oxirane ring opening with primary amines resulted in N-substituted aminodiols. The regioselective ring closure of these aminodiols with formaldehyde was also investigated. Benzylation of isopulegol furnished O-benzyl-protected isopulegol, which was transformed into aminoalcohols via epoxidation and ring opening of the corresponding epoxides. First benzyl-protected isopulegol was subjected to hydroxylation and epoxidation, then aminolysis of the served oxiranes delivered aminodiols. On the other hand, (-)-isopulegol was oxidised to diol, which was again converted into both dibenzyl- and monobenzyl-protected diol derivatives. The products were transformed into aminoalcohols and aminodiols, respectively, by aminolysis of their epoxides. The ring opening of epoxides, derived from diols with primary amines was also performed producing aminotriols. Dihydroxylation of (-)-isopulegol or derivatives with OsO4/NMO gave isopulegol-based di-, tri- and tetraols. The antimicrobial activity and antioxidant property, measuring DPPH˙ free radical scavenging activity of aminodiol and aminotriol derivatives as well as di-, tri- and tetraols were also explored. In addition, structure-activity relationships were examined from the aspects of substituent effects and stereochemistry on the aminodiol and aminotriol systems.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ciesla L. M. Wojtunik-Kulesza K. A. Oniszczuk A. Waksmundzka-Hajnos M. Antioxidant synergism and antagonism between selected monoterpenes using the 2,2-diphenyl-1-picrylhydrazyl method: Antioxidant synergism and antagonism between selected monoterpenes. Flavour Fragrance J. 2016;31:412–419. doi: 10.1002/ffj.3330. - DOI
-
- Wen Xu Y. Effects of salicylic acid on monoterpene production and antioxidant systems in Houttuynia cordata. Afr. J. Biotechnol. 2012;11:1364–1372.
LinkOut - more resources
Full Text Sources

