Amino-functionalized magnetic chitosan beads to enhance immobilization of potassium copper hexacyanoferrate for selective Cs+ removal and facile recovery
- PMID: 35517610
- PMCID: PMC9059498
- DOI: 10.1039/c8ra09386e
Amino-functionalized magnetic chitosan beads to enhance immobilization of potassium copper hexacyanoferrate for selective Cs+ removal and facile recovery
Abstract
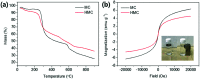
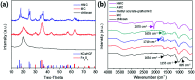
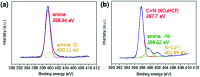
Potassium copper hexacyanoferrate (KCuHCF)-incorporated magnetic chitosan beads (HMC) were synthesized for both selective Cs+ removal in aqueous solutions and facile recovery of the spent adsorbent. To disperse and immobilize large amounts of the KCuHCF, methyl acrylate and diethylenetriamine were sequentially grafted onto the one-step synthesized magnetic chitosan beads. The additional introduction of amino functionality led to the enriched Cu2+ ions on the bead surface to incorporate KCuHCF into the grafting matrix. Consequently, the HMC exhibited a high Cs+ capacity calculated to be 136.47 mg g-1 from the Langmuir model, and the equilibrium was established within 4 h. Moreover, the HMC exhibited excellent stability in a wide pH range from 4 to 11 and an outstanding Cs+ selectivity (>97%) in seawater (1.11 mg L-1 Cs+). From a practical point of view, the HMC was stable during five successive adsorption cycles and easily recovered by magnets, enabling continuous operation to decontaminate a large volume of wastewater.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Highly effective Cs+ removal by turbidity-free potassium copper hexacyanoferrate-immobilized magnetic hydrogels.J Hazard Mater. 2017 Oct 15;340:130-139. doi: 10.1016/j.jhazmat.2017.06.066. Epub 2017 Jun 28. J Hazard Mater. 2017. PMID: 28715736
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Bio-Inspired Preparation of Clay-Hexacyanoferrate Composite Hydrogels as Super Adsorbents for Cs.ACS Appl Mater Interfaces. 2020 Jul 22;12(29):33173-33185. doi: 10.1021/acsami.0c06598. Epub 2020 Jul 7. ACS Appl Mater Interfaces. 2020. PMID: 32531151
-
Sodium-copper hexacyanoferrate-functionalized magnetic nanoclusters for the highly efficient magnetic removal of radioactive caesium from seawater.Water Res. 2017 Nov 15;125:81-90. doi: 10.1016/j.watres.2017.08.037. Epub 2017 Aug 17. Water Res. 2017. PMID: 28834769
-
Hybrid porous magnetic bentonite-chitosan beads for selective removal of radioactive cesium in water.J Hazard Mater. 2019 Jan 15;362:160-169. doi: 10.1016/j.jhazmat.2018.08.067. Epub 2018 Sep 9. J Hazard Mater. 2019. PMID: 30236936
Cited by
-
Chitosan@Carboxymethylcellulose/CuO-Co2O3 Nanoadsorbent as a Super Catalyst for the Removal of Water Pollutants.Gels. 2022 Feb 3;8(2):91. doi: 10.3390/gels8020091. Gels. 2022. PMID: 35200472 Free PMC article.
-
Aspergillus tamarii mediated green synthesis of magnetic chitosan beads for sustainable remediation of wastewater contaminants.Sci Rep. 2022 Jun 13;12(1):9742. doi: 10.1038/s41598-022-13534-1. Sci Rep. 2022. PMID: 35697833 Free PMC article.
References
-
- Wang Y.-X. Li J.-R. Yang J.-C. E. Yuan B. Fu M.-L. RSC Adv. 2015;5:91431–91435. doi: 10.1039/C5RA16205J. - DOI
-
- International Energy Agency, Key World Energy Statistics, http://www.iea.org/publications/freepublications/publication/KeyWorld201...
-
- Baik S. Zhang H. Kim Y. K. Harbottle D. Lee J. W. RSC Adv. 2017;7:54546–54553. doi: 10.1039/C7RA09541D. - DOI
-
- Zhang H. Tangparitkul S. Hendry B. Harper J. Kon Kim Y. Hunter T. N. Lee J. W. Harbottle D. Chem. Eng. J. 2019;355:797–804. doi: 10.1016/j.cej.2018.07.135. - DOI
LinkOut - more resources
Full Text Sources

