Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N-H compounds
- PMID: 35517670
- PMCID: PMC9062143
- DOI: 10.1039/c9ra00929a
Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N-H compounds
Abstract
Carboxylic acids and their derivatives are ubiquitous compounds in organic chemistry, and are widely commercially available in a large structural variety. Recently, carboxylic acids have been frequently used as non-toxic and environmentally benign alternatives to traditional organohalide coupling partners in various carbon-carbon and carbon-heteroatom cross-coupling reactions. Along this line, several methods have been reported for the synthesis of nitrogen-containing organic compounds through decarboxylative cross-coupling reactions between carboxylic acids and N-H compounds. This review focuses on recent advances and discoveries on these reactions with special attention on the mechanistic aspects of the reactions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

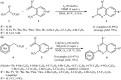


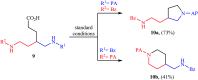
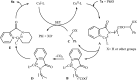

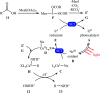

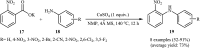
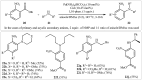


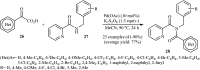
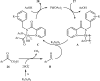









References
-
- McGrath N. A. Brichacek M. Njardarson J. T. J. Chem. Educ. 2010;87:1348–1349. doi: 10.1021/ed1003806. - DOI
-
- Lawrence S. A., Amines: synthesis, properties and applications, Cambridge University Press, Cambridge, UK, 2004
- Fujiwara T. O'Hagan D. J. Fluorine Chem. 2014;167:16–29. doi: 10.1016/j.jfluchem.2014.06.014. - DOI
- Devendar P. Yang G.-F. Top. Curr. Chem. 2017;375:82. doi: 10.1007/s41061-017-0169-9. - DOI - PubMed
- Frings M. Bolm C. Blum A. Gnamm C. Eur. J. Med. Chem. 2017;126:225–245. doi: 10.1016/j.ejmech.2016.09.091. - DOI - PubMed
-
- Fache F. Schulz E. Tommasino M. L. Lemaire M. Chem. Rev. 2000;100:2159–2232. doi: 10.1021/cr9902897. - DOI - PubMed
- El Kadib A. ChemSusChem. 2015;8:217–244. doi: 10.1002/cssc.201402718. - DOI - PubMed
- Evano G. Coste A. Jouvin K. Angew. Chem., Int. Ed. 2010;49:2840–2859. doi: 10.1002/anie.200905817. - DOI - PubMed
- Arshadi S. Vessally E. Edjlali L. Ghorbani-Kalhor E. Hosseinzadeh-Khanmiri R. RSC Adv. 2017;7:13198–13211. doi: 10.1039/C7RA00746A. - DOI - PMC - PubMed
- Bhattacharyya N. Jha S. Jha S. Bhutia T. Y. Adhikary G. Int. J. Appl. Pharm. 2012;4:295–304.
Publication types
LinkOut - more resources
Full Text Sources

