A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors
- PMID: 35517682
- PMCID: PMC9062009
- DOI: 10.1039/c8ra09577a
A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors
Abstract
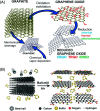
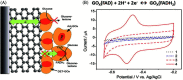
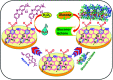
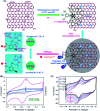
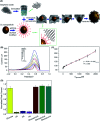
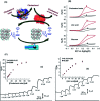
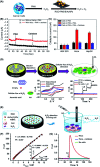
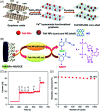
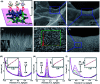
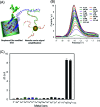
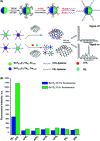
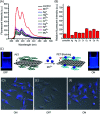
Biosensors with high sensitivity, selectivity and a low limit of detection, reaching nano/picomolar concentrations of biomolecules, are important to the medical sciences and healthcare industry for evaluating physiological and metabolic parameters. Over the last decade, different nanomaterials have been exploited to design highly efficient biosensors for the detection of analyte biomolecules. The discovery of graphene has spectacularly accelerated research on fabricating low-cost electrode materials because of its unique physical properties, including high specific surface area, high carrier mobility, high electrical conductivity, flexibility, and optical transparency. Graphene and its oxygenated derivatives, including graphene oxide (GO) and reduced graphene oxide (rGO), are becoming an important class of nanomaterials in the field of biosensors. The presence of oxygenated functional groups makes GO nanosheets strongly hydrophilic, facilitating chemical functionalization. Graphene, GO and rGO nanosheets can be easily combined with various types of inorganic nanoparticles, including metals, metal oxides, semiconducting nanoparticles, quantum dots, organic polymers and biomolecules, to create a diverse range of graphene-based nanocomposites with enhanced sensitivity for biosensor applications. This review summarizes the advances in two-dimensional (2D) and three-dimensional (3D) graphene-based nanocomposites as emerging electrochemical and fluorescent biosensing platforms for the detection of a wide range of biomolecules with enhanced sensitivity, selectivity and a low limit of detection. The biofunctionalization and nanocomposite formation processes of graphene-based materials and their unique properties, surface functionalization, enzyme immobilization strategies, covalent immobilization, physical adsorption, biointeractions and direct electron transfer (DET) processes are discussed in connection with the design and fabrication of biosensors. The enzymatic and nonenzymatic reactions on graphene-based nanocomposite surfaces for glucose- and cholesterol-related electrochemical biosensors are analyzed. This review covers a very broad range of graphene-based electrochemical and fluorescent biosensors for the detection of glucose, cholesterol, hydrogen peroxide (H2O2), nucleic acids (DNA/RNA), genes, enzymes, cofactors nicotinamide adenine dinucleotide (NADH) and adenosine triphosphate (ATP), dopamine (DA), ascorbic acid (AA), uric acid (UA), cancer biomarkers, pathogenic microorganisms, food toxins, toxic heavy metal ions, mycotoxins, and pesticides. The sensitivity and selectivity of graphene-based electrochemical and fluorescent biosensors are also examined with respect to interfering analytes present in biological systems. Finally, the future outlook for the development of graphene based biosensing technology is outlined.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


































References
-
- Malhotra B. D. Chaubey A. Biosensors for Clinical Diagnostics Industry. Sens. Actuators, B. 2003;91:117–127. doi: 10.1016/S0925-4005(03)00075-3. - DOI
-
- Velasco-Garcia M. N. Mottram T. Biosensor Technology Addressing Agricultural Problems. Biosyst. Eng. 2003;84:1–12. doi: 10.1016/S1537-5110(02)00236-2. - DOI
-
- Rodriguezmozaz S. Alda M. Marco M. Barcelo D. Biosensors for Environmental Monitoring A Global Perspective. Talanta. 2005;65:291–297. - PubMed
-
- Su S. Chen S. Fan C. Recent Advances in Two-Dimensional Nanomaterials-Based Electrochemical Sensors for Environmental Analysis. Green Energy & Environment. 2018;3:97–106.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

