Near-Infrared Fluorescence Imaging of EGFR-Overexpressing Tumors in the Mouse Xenograft Model Using scFv-IRDye800CW and Cetuximab-IRDye800CW
- PMID: 35517713
- PMCID: PMC9042373
- DOI: 10.1155/2022/9589820
Near-Infrared Fluorescence Imaging of EGFR-Overexpressing Tumors in the Mouse Xenograft Model Using scFv-IRDye800CW and Cetuximab-IRDye800CW
Abstract
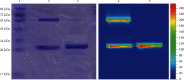
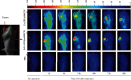
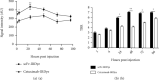
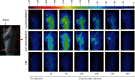
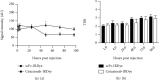
EGFR (epidermal growth factor receptor) is overexpressed in a variety of human cancers (including squamous cell carcinoma of head and neck, colon cancer, and some breast cancers) and therefore is regarded as an ideal target for cancer therapy or imaging purposes. In the current study, we produced a scFv-based near-infrared probe (called cet.Hum.scFv-IRDye-800CW) and evaluated its ability in recognizing and imaging of EGFR-overexpressing tumors in a mouse model. Like the molecular probe consisting of its parental antibody (cetuximab, an FDA-approved monoclonal antibody) and IRD800CW, cet.Hum.scFv-IRDye-800CW was able to recognize EGFR-overexpressing tumors in mice. cet.Hum.scFv-IRDye-800CW was found to be superior to the cetuximab-based probe in imaging of mouse tumors. The tumor-to-background ratio and blood clearance rate were higher when cet.Hum.scFv-IRDye-800CW was used as an imaging probe.
Copyright © 2022 Abolfazl Amini et al.
Conflict of interest statement
The authors declare that they have no conflicts of interests.
Figures


Similar articles
-
Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma.Mol Imaging Biol. 2013 Dec;15(6):722-9. doi: 10.1007/s11307-013-0652-9. Mol Imaging Biol. 2013. PMID: 23715932 Free PMC article.
-
IRDye 800CW-Anti-epidermal growth factor receptor nanobody 7D12.2012 Apr 6 [updated 2012 Jul 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Apr 6 [updated 2012 Jul 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22787693 Free Books & Documents. Review.
-
Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW.Br J Neurosurg. 2015;29(6):850-8. doi: 10.3109/02688697.2015.1056090. Epub 2015 Jun 15. Br J Neurosurg. 2015. PMID: 26073144 Free PMC article.
-
Near infrared imaging of epidermal growth factor receptor positive xenografts in mice with domain I/II specific antibody fragments.Theranostics. 2019 Jan 30;9(4):974-985. doi: 10.7150/thno.30835. eCollection 2019. Theranostics. 2019. PMID: 30867810 Free PMC article.
-
Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.Drugs Today (Barc). 2005 Feb;41(2):107-27. doi: 10.1358/dot.2005.41.2.882662. Drugs Today (Barc). 2005. PMID: 15821783 Review.
Cited by
-
Tumour induction in BALB/c mice for imaging studies: An improved protocol.J Cell Mol Med. 2023 Jul;27(13):1880-1886. doi: 10.1111/jcmm.17792. Epub 2023 May 29. J Cell Mol Med. 2023. PMID: 37246626 Free PMC article.
-
Rising sun or strangled in the cradle? A narrative review of near-infrared fluorescence imaging-guided surgery for pancreatic tumors.Int J Surg. 2024 Dec 1;110(12):7929-7947. doi: 10.1097/JS9.0000000000001676. Int J Surg. 2024. PMID: 38768476 Free PMC article. Review.
-
Correlation between 18F-FDG PET/MR parameters with the expression level of epidermal growth factor receptor and the diagnostic value of PET/MR in head and neck squamous cell carcinoma.Heliyon. 2023 Mar 22;9(4):e14822. doi: 10.1016/j.heliyon.2023.e14822. eCollection 2023 Apr. Heliyon. 2023. PMID: 37089359 Free PMC article.
-
Near-infrared fluorescent molecular probes with cetuximab in the in vivo fluorescence imaging for epithelial ovarian cancer.J Ovarian Res. 2024 Nov 14;17(1):225. doi: 10.1186/s13048-024-01547-5. J Ovarian Res. 2024. PMID: 39543737 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

