Dose Optimisation of Posaconazole and Therapeutic Drug Monitoring in Pediatric Patients
- PMID: 35517786
- PMCID: PMC9061949
- DOI: 10.3389/fphar.2022.833303
Dose Optimisation of Posaconazole and Therapeutic Drug Monitoring in Pediatric Patients
Abstract
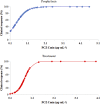
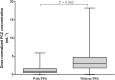
Experience in the clinical use of posaconazole (PCZ) in pediatric patients is limited, and no specific dose recommendations exist. This study aimed to investigate an appropriate dosing regimen, and assess the exposure-response relationships of PCZ in children. We reviewed the medical records of inpatients aged <18 years who subjected to PCZ concentrations monitoring. Clinical data, PCZ dosing and monitoring data were collected. A total of 375 PCZ trough concentrations (C min) from 105 pediatric patients were included. For children receiving PCZ for prophylaxis, the median doses required to achieve the therapeutic range at the ages of <6, 6-12 and >12 years were 14.80, 14.52 and 12.90 mg/kg/day, respectively (p = 0.001); and for those receiving PCZ for treatment, the median doses were 23.50, 20.96 and 15.38 mg/kg/day, respectively (p = 0.001). Among children taking PCZ for prophylaxis, 12% developed a proven or probable breakthrough IFIs; the median PCZ concentrations were significantly lower than those children with successful treatment response (0.43 versus 1.20 μg mL-1; p < 0.001). 79.2% patients taking PCZ for treatment had a positive clinical response, and the median PCZ concentrations were significantly higher than those children with disease progression (1.06 versus 0.53 μg mL-1; p = 0.024). No association between C min values and hepatotoxicity was observed. Factors such as age, CRP, ALT and co-administration with proton pump inhibitors exhibited significant effects on PCZ C min. It is necessary to adjust the dosing regimens based on PCZ C min to individualize antifungal therapy and provide guidelines for dose adjustment in children.
Keywords: invasive fungal infections; pediatric patients; pharmacokinetics; posaconazole; therapeutic drug monitoring.
Copyright © 2022 Jia, Zhang, Qin, Wang, Liu, Yang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Arrieta A. C., Sung L., Bradley J. S., Zwaan C. M., Gates D., Waskin H., et al. (2019). A Non-randomized Trial to Assess the Safety, Tolerability, and Pharmacokinetics of Posaconazole Oral Suspension in Immunocompromised Children with Neutropenia. PLoS One 14, e0212837. 10.1371/journal.pone.0212837 - DOI - PMC - PubMed
-
- Bernardo V., Miles A., Fernandez A. J., Liverman R., Tippett A., Yildirim I. (2020). Initial Posaconazole Dosing to Achieve Therapeutic Serum Posaconazole Concentrations Among Children, Adolescents, and Young Adults Receiving Delayed-Release Tablet and Intravenous Posaconazole. Pediatr. Transpl. 24, e13777. 10.1111/petr.13777 - DOI - PubMed
-
- Chau M. M., Kong D. C., van Hal S. J., Urbancic K., Trubiano J. A., Cassumbhoy M., et al. (2014). Consensus Guidelines for Optimising Antifungal Drug Delivery and Monitoring to Avoid Toxicity and Improve Outcomes in Patients with Haematological Malignancy, 2014. Intern. Med. J. 44, 1364–1388. 10.1111/imj.12600 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

