Folic Acid-Decorated pH-Responsive Nanoniosomes With Enhanced Endocytosis for Breast Cancer Therapy: In Vitro Studies
- PMID: 35517801
- PMCID: PMC9065559
- DOI: 10.3389/fphar.2022.851242
Folic Acid-Decorated pH-Responsive Nanoniosomes With Enhanced Endocytosis for Breast Cancer Therapy: In Vitro Studies
Abstract
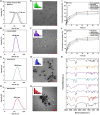
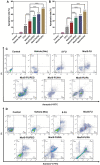
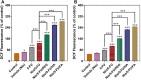
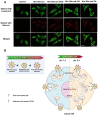
Breast cancer is the most common invasive cancer in women and the second leading cause of cancer death in women after lung cancer. The purpose of this study is a targeted delivery toward in vitro (on MCF7 and 4T1 breast cancer cell lines) through niosomes-based nanocarriers. To this end, different bioactive molecules, including hyaluronic acid (HA), folic acid (FA), and polyethylene glycol (PEG), were used and compared for surface modification of niosomes to enhance endocytosis. FA-functionalized niosomes (Nio/5-FU/FA) were able to increase cell cytotoxicity and reduce cell migration and invasion compared to PEG-functionalized niosomes (Nio/5-FU/PEG), and HA-functionalized niosomes (Nio/5-FU/HA) groups in MCF-7 and 4T1 cell lines. Although the Nio/5-FU/PEG and Nio/5-FU/HA demonstrated MCF7 cell uptake, the Nio/5-FU/FA exhibited the most preponderant endocytosis in pH 5.4. Remarkably, in this study 5-FU loaded niosomes (nonionic surfactant-based vesicles) were decorated with various bioactive molecules (FA, PEG, or HA) to compare their ability for breast cancer therapy. The fabricated nanoformulations were readily taken up by breast cancer cells (in vitro) and demonstrated sustained drug release characteristics, inducing cell apoptosis. Overall, the comprehensive comparison between different bioactive molecules-decorated nanoniosomes exhibited promising results in finding the best nano formulated candidates for targeted delivery of drugs for breast cancer therapy.
Keywords: 5-FU; breast cancer; endocytosis; folic acid; hyaluronic acid; niosome.
Copyright © 2022 Rezaei, Rezaei, Karimifard, Mahmoudi Beram, Dakkali, Heydari, Afshari-Behbahanizadeh, Mostafavi, Bokov, Ansari, Farasati Far, Akbarzadeh and Chaiyasut.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Akbari J., Saeedi M., Enayatifard R., Morteza-Semnani K., Hassan Hashemi S. M., Babaei A., et al. (2020). Curcumin Niosomes (Curcusomes) as an Alternative to Conventional Vehicles: A Potential for Efficient Dermal Delivery. J. Drug Deliv. Sci. Tech. 60, 102035. 10.1016/j.jddst.2020.102035 - DOI
-
- Akbarzadeh I., Tavakkoli Yaraki M., Ahmadi S., Chiani M., Nourouzian D. (2020a). Folic Acid-Functionalized Niosomal Nanoparticles for Selective Dual-Drug Delivery into Breast Cancer Cells: An In-Vitro Investigation. Adv. Powder Tech. 31 (9), 4064–4071. 10.1016/j.apt.2020.08.011 - DOI
-
- Akbarzadeh I., Tavakkoli Yaraki M., Bourbour M., Noorbazargan H., Lajevardi A., Sadat Shilsar S. M., et al. (2020b). Optimized Doxycycline-Loaded Niosomal Formulation for Treatment of Infection-Associated Prostate Cancer: An In-Vitro Investigation. J. Drug Deliv. Sci. Tech. 57, 101715. 10.1016/j.jddst.2020.101715 - DOI
-
- Alemi A., Zavar Reza J., Haghiralsadat F., Zarei Jaliani H., Haghi Karamallah M., Hosseini S. A., et al. (2018). Paclitaxel and Curcumin Coadministration in Novel Cationic PEGylated Niosomal Formulations Exhibit Enhanced Synergistic Antitumor Efficacy. J. Nanobiotechnology 16 (1), 28–20. 10.1186/s12951-018-0351-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

