Drug-Induced Severe Cutaneous Adverse Reactions: Insights Into Clinical Presentation, Immunopathogenesis, Diagnostic Methods, Treatment, and Pharmacogenomics
- PMID: 35517811
- PMCID: PMC9065683
- DOI: 10.3389/fphar.2022.832048
Drug-Induced Severe Cutaneous Adverse Reactions: Insights Into Clinical Presentation, Immunopathogenesis, Diagnostic Methods, Treatment, and Pharmacogenomics
Abstract
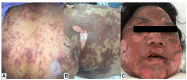
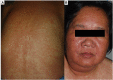
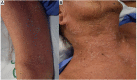
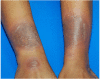
SCARs are rare and life-threatening hypersensitivity reactions. In general, the increased duration of hospital stays and the associated cost burden are common issues, and in the worst-case scenario, they can result in mortality. SCARs are delayed T cell-mediated hypersensitivity reactions. Recovery can take from 2 weeks to many months after dechallenging the culprit drugs. Genetic polymorphism of the HLA genes may change the selection and presentation of antigens, allowing toxic drug metabolites to initiate immunological reactions. However, each SCARs has a different onset latency period, clinical features, or morphological pattern. This explains that, other than HLA mutations, other immuno-pathogenesis may be involved in drug-induced severe cutaneous reactions. This review will discuss the clinical morphology of various SCARs, various immune pathogenesis models, diagnostic criteria, treatments, the association of various drug-induced reactions and susceptible alleles in different populations, and the successful implementation of pharmacogenomics in Thailand for the prevention of SCARs.
Keywords: PGx implementation; SCARs; Thailand; immunopathogenesis of SCARs; pharmacogenomics; risk factors.
Copyright © 2022 Tempark, John, Rerknimitr, Satapornpong and Sukasem.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Genotyping HLA alleles to predict the development of Severe cutaneous adverse drug reactions (SCARs): state-of-the-art.Expert Opin Drug Metab Toxicol. 2021 Sep;17(9):1049-1064. doi: 10.1080/17425255.2021.1946514. Epub 2021 Jul 8. Expert Opin Drug Metab Toxicol. 2021. PMID: 34148467 Review.
-
Analysis of HLA-B Allelic Variation and IFN-γ ELISpot Responses in Patients with Severe Cutaneous Adverse Reactions Associated with Drugs.J Allergy Clin Immunol Pract. 2019 Jan;7(1):219-227.e4. doi: 10.1016/j.jaip.2018.05.004. Epub 2018 May 22. J Allergy Clin Immunol Pract. 2019. PMID: 29800753
-
A Study of Cutaneous Adverse Drug Reactions at a Tertiary Care Center in Andhra Pradesh, India.Cureus. 2023 Apr 14;15(4):e37596. doi: 10.7759/cureus.37596. eCollection 2023 Apr. Cureus. 2023. PMID: 37197134 Free PMC article.
-
Current understanding of genetic associations with delayed hypersensitivity reactions induced by antibiotics and anti-osteoporotic drugs.Front Pharmacol. 2023 Apr 26;14:1183491. doi: 10.3389/fphar.2023.1183491. eCollection 2023. Front Pharmacol. 2023. PMID: 37180708 Free PMC article. Review.
-
A Comprehensive Review of HLA and Severe Cutaneous Adverse Drug Reactions: Implication for Clinical Pharmacogenomics and Precision Medicine.Pharmaceuticals (Basel). 2021 Oct 25;14(11):1077. doi: 10.3390/ph14111077. Pharmaceuticals (Basel). 2021. PMID: 34832859 Free PMC article. Review.
Cited by
-
Web application for assisting non-dermatology physicians in learning and managing patients with common cutaneous adverse drug reactions: a multicenter randomized controlled trial.Ann Med. 2024 Dec;56(1):2422573. doi: 10.1080/07853890.2024.2422573. Epub 2024 Oct 30. Ann Med. 2024. PMID: 39473307 Free PMC article. Clinical Trial.
-
Gabapentin for refractory pruritus in severe cutaneous adverse reactions.JAAD Case Rep. 2025 Feb 21;59:4-5. doi: 10.1016/j.jdcr.2025.01.026. eCollection 2025 May. JAAD Case Rep. 2025. PMID: 40225090 Free PMC article. No abstract available.
-
Antibiotic-induced severe cutaneous adverse reactions: a single-center retrospective study over ten years.Front Immunol. 2024 Jul 18;15:1415830. doi: 10.3389/fimmu.2024.1415830. eCollection 2024. Front Immunol. 2024. PMID: 39091503 Free PMC article.
-
Alemtuzumab-induced petechiae and epistaxis in a patient with relapsing-remitting multiple sclerosis: A case report.Clin Case Rep. 2023 Nov 25;11(11):e8143. doi: 10.1002/ccr3.8143. eCollection 2023 Nov. Clin Case Rep. 2023. PMID: 38028047 Free PMC article.
-
[Frequency of cutaneous drug reactions in the outpatient dermatology clinic at the National Institute of Cardiology Ignacio Chávez over a ten-year period].Arch Cardiol Mex. 2024 Oct 10;95(1):59-68. doi: 10.24875/ACM.24000107. Arch Cardiol Mex. 2024. PMID: 39388648 Free PMC article. Spanish.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

