Fatty Acid β-Oxidation in Kidney Diseases: Perspectives on Pathophysiological Mechanisms and Therapeutic Opportunities
- PMID: 35517820
- PMCID: PMC9065343
- DOI: 10.3389/fphar.2022.805281
Fatty Acid β-Oxidation in Kidney Diseases: Perspectives on Pathophysiological Mechanisms and Therapeutic Opportunities
Abstract
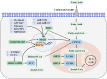
The kidney is a highly metabolic organ and requires a large amount of ATP to maintain its filtration-reabsorption function, and mitochondrial fatty acid β-oxidation serves as the main source of energy to meet its functional needs. Reduced and inefficient fatty acid β-oxidation is thought to be a major mechanism contributing to kidney diseases, including acute kidney injury, chronic kidney disease and diabetic nephropathy. PPARα, AMPK, sirtuins, HIF-1, and TGF-β/SMAD3 activation have all been shown to play key roles in the regulation of fatty acid β-oxidation in kidney diseases, and restoration of fatty acid β-oxidation by modulation of these molecules can ameliorate the development of such diseases. Here, we disentangle the lipid metabolism regulation properties and potential mechanisms of mesenchymal stem cells and their extracellular vesicles, and emphasize the role of mesenchymal stem cells on lipid metabolism. This review aims to highlight the important role of fatty acid β-oxidation in the progression of kidney diseases, and to explore the fatty acid β-oxidation effects and therapeutic potential of mesenchymal stem cells for kidney diseases.
Keywords: acute kidney injury; chronic kidney disease; diabetic nephropathy; fatty acid β-oxidation; mesenchymal stem cell therapy.
Copyright © 2022 Gao and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Blanquart C., Barbier O., Fruchart J. C., Staels B., Glineur C. (2002). Peroxisome Proliferator-Activated Receptor Alpha (PPARalpha ) Turnover by the Ubiquitin-Proteasome System Controls the Ligand-Induced Expression Level of its Target Genes. J. Biol. Chem. 277, 37254–37259. 10.1074/jbc.M110598200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

