TLRs-JNK/ NF-κB Pathway Underlies the Protective Effect of the Sulfide Salt Against Liver Toxicity
- PMID: 35517830
- PMCID: PMC9065287
- DOI: 10.3389/fphar.2022.850066
TLRs-JNK/ NF-κB Pathway Underlies the Protective Effect of the Sulfide Salt Against Liver Toxicity
Abstract
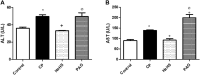
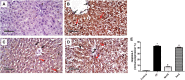
Hydrogen sulfide (H2S) is an endogenously gas transmitter signaling molecule with known antioxidant, anti-inflammatory, and cytoprotective properties. Although accumulating evidence shows the therapeutic potential of H2S in various hepatic diseases, its role in cyclophosphamide (CP)-induced hepatotoxicity remains elusive. The present study was undertaken to investigate the impact of endogenous and exogenous H2S on toll-like receptors (TLRs)-mediated inflammatory response and apoptosis in CP-induced hepatotoxicity. Either an H2S donor (NaHS (100 μM/kg) or an H2S blocker [dl-propargylglycine (PAG) (30 mg/kg, i. p.)], was administered for 10 days before a single ip injection of CP (200 mg/kg). NaHS attenuated conferred hepatoprotection against CP-induced toxicity, significantly decreasing serum hepatic function tests and improving hepatic histopathology. Additionally, NaHS-treated rats exhibited antioxidant activity in liver tissues compared with the CP group. The upregulated hepatic levels of TLR2/4 and their downstream signaling molecules including c-Jun N-terminal kinase (JNK) and nuclear factor-kappa B (NF-κB) were also suppressed by NaHS protective treatment. NaHS showed anti-inflammatory and antiapoptotic effects; reducing hepatic level tumor necrosis factor-alpha (TNF-α) and caspase-3 expression. Interestingly, the cytotoxic events induced in CP-treated rats were not significantly altered upon the blocking of endogenous H2S. Taken together, the present study suggested that exogenously applied H2S rather than the endogenously generated H2S, displayed a hepatoprotective effect against CP-induced hepatotoxicity that might be mediated by TLRs-JNK/NF-κB pathways.
Keywords: hepatotoxicity; hydrogen sulfide; inflammatory response; oxidative stress; toll-like receptors.
Copyright © 2022 Abdel-latif, Heeba, Hassanin, Waz and Amin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aladaileh S. H., Abukhalil M. H., Saghir S. A. M., Hanieh H., Alfwuaires M. A., Almaiman A. A., et al. (2019). Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity. Biomolecules 9 (8), 346. 10.3390/biom9080346 - DOI - PMC - PubMed
-
- ALHaithloul H. A. S., Alotaibi M. F., Bin-Jumah M., Elgebaly H., Mahmoud A. M. (2019). Olea Europaea Leaf Extract Up-Regulates Nrf2/ARE/HO-1 Signaling and Attenuates Cyclophosphamide-Induced Oxidative Stress, Inflammation and Apoptosis in Rat Kidney. Biomed. Pharmacother. 111, 676–685. 10.1016/j.biopha.2018.12.112 - DOI - PubMed
-
- Caglayan C., Temel Y., Kandemir F. M., Yildirim S., Kucukler S. (2018). Naringin Protects against Cyclophosphamide-Induced Hepatotoxicity and Nephrotoxicity through Modulation of Oxidative Stress, Inflammation, Apoptosis, Autophagy, and DNA Damage. Environ. Sci. Pollut. Res. Int. 25 (21), 20968–20984. 10.1007/s11356-018-2242-5 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

