Regioselective C-3-alkylation of quinoxalin-2(1 H)-ones via C-N bond cleavage of amine derived Katritzky salts enabled by continuous-flow photoredox catalysis
- PMID: 35517836
- PMCID: PMC9053435
- DOI: 10.1039/d2ra00753c
Regioselective C-3-alkylation of quinoxalin-2(1 H)-ones via C-N bond cleavage of amine derived Katritzky salts enabled by continuous-flow photoredox catalysis
Abstract
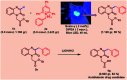
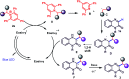
An efficient, transition metal-free visible-light-driven continuous-flow C-3-alkylation of quinoxalin-2(1H)-ones has been demonstrated by employing Katritzky salts as alkylating agents in the presence of eosin-y as a photoredox catalyst and DIPEA as a base at room temperature. The present protocol was accomplished by utilizing abundant and inexpensive alkyl amine (both primary and secondary alkyl) and as well as this a few amino acid feedstocks were converted into their corresponding redox-active pyridinium salts and subsequently into alkyl radicals. A wide variety of C-3-alkylated quinoxalin-2(1H)-ones were synthesized in moderate to high yields. Further this environmentally benign protocol is carried out in a PFA (Perfluoroalkoxy alkane) capillary based micro reactor under blue LED irradiation, enabling excellent yields (72% to 91%) and shorter reaction times (0.81 min) as compared to a batch system (16 h).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Carta A. Piras S. Loriga G. Paglietti G. Mini-Rev. Med. Chem. 2006;6:1179–1200. - PubMed
- Liu R. Huang Z.-H. Murray M. G. Guo X.-Y. Liu G. J. Med. Chem. 2011;54:5747–5768. - PubMed
- Galal S. A. Khairat S. H. M. Ragab F. A. F. Abdelsamie A. S. Ali M. M. Sliman S. M. Mortier J. Wolber G. El Diwani H. Eur. J. Med. Chem. 2014;86:122–132. - PubMed
- Qin X. Hao X. Han H. Zhu S. Yang Y. Wu B. Hussain S. Parveen S. Jing C. Ma B. Zhu C. J. Med. Chem. 2015;58:1254–1267. - PubMed
-
- Udilova N. Kozlov A. V. Bieberschulte W. Frei K. Ehrenberger K. Nohl H. Biochem. Pharmacol. 2003;65:59–65. - PubMed
- Dudash J. Zhang Y. Moore J. B. Look R. Liang Y. Beavers M. P. Conway B. R. Rybczynski P. J. Demarest K. T. Bioorg. Med. Chem. Lett. 2005;15:4790–4793. - PubMed
- Abu-Hashem A. A. Gouda M. A. Badria F. A. Eur. J. Med. Chem. 2010;45:1976–1981. - PubMed
- Hussain S. Parveen S. Hao X. Zhang S. Wang W. Qin X. Yang Y. Chen X. Zhu S. Zhu C. Ma B. Eur. J. Med. Chem. 2014;80:383–392. - PubMed
- Issa D. A. E. Habib N. S. Abdel Wahab A. E. MedChemComm. 2015;6:202–211.
-
- Carrër A. Brion J. D. Messaoudi S. Alami M. Org. Lett. 2013;15:5606–5609. - PubMed
- Yin K. Zhang R. Org. Lett. 2017;19:1530–1533. - PubMed
- Yuan J. Liu S. Qu L. Adv. Synth. Catal. 2017;359:4197–4207.
- Paul S. Ha J. H. Park G. E. Lee Y. R. Adv. Synth. Catal. 2017;359:1515–1521.
- Zeng X. Liu C. Wang X. Zhang J. Wang X. Hu Y. Org. Biomol. Chem. 2017;15:8929–8935. - PubMed
- Yuan J.-W. Fu J.-H. Liu S.-N. Xiao Y.-M. Mao P. Qu L.-B. Org. Biomol. Chem. 2018;16:3203–3212. - PubMed
- Kim Y. Kim D. Y. Tetrahedron Lett. 2018;59:2443–2446.
- Gao M. Li Y. Xie L. Chauvin R. Cui X. Chem. Commun. 2016;52:2846–2849. - PubMed
- Xie L.-Y. Chen Y.-L. Qin L. Wen Y. Xie J.-W. Tan J.-X. Huang Y. Cao Z. He W.-M. Org. Chem. Front. 2019;6:3950–3955.
- Zhou J. Zhou P. Zhao T. Ren Q. Li J. Adv. Synth. Catal. 2019;361:5371–5382.
- Hoang T. T. To T. A. Cao V. T. T. Nguyen A. T. Nguyen T. T. Phan N. T. S. Catal. Commun. 2017;101:20–25.
- Wei W. Wang L. Bao P. Shao Y. Yue H. Yang D. Yang X. Zhao X. Wang H. Org. Lett. 2018;20:7125–7130. - PubMed
- Li K.-J. Xu K. Liu Y.-G. Zeng C.-C. Sun B.-G. Adv. Synth. Catal. 2019;361:1033–1041.
- Yuan J. Zhu J. Fu J. Yang L. Xiao Y. Mao P. Du X. Qu L. Org. Chem. Front. 2019;6:925–935.
- Wang L. Zhang Y. Li F. Hao X. Zhang H.-Y. Zhao J. Adv. Synth. Catal. 2018;360:3969–3977.
- Sun M. Wang L. Zhao L. Wang Z. Li P. ChemCatChem. 2020;12:5261–5268.
-
- Hu L. Q. Yuan J. W. Fu J. H. Zhang T. T. Gao L. L. Xiao Y. M. Mao P. Qu L. B. Eur. J. Org. Chem. 2018:4113–4120.
- Yuan J. Fu J. Yin J. Dong Z. Xiao Y. Mao P. Qu L. Org. Chem. Front. 2018;5:2820–2828.
- Fu J. Yuan J. Zhang Y. Xiao Y. Mao P. Diao X. Qu L. Org. Chem. Front. 2018;5:3382–3390.
LinkOut - more resources
Full Text Sources