Functionalization of MOF-5 with mono-substituents: effects on drug delivery behavior
- PMID: 35517920
- PMCID: PMC9057024
- DOI: 10.1039/d0ra06106a
Functionalization of MOF-5 with mono-substituents: effects on drug delivery behavior
Abstract
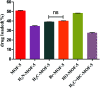
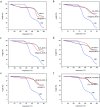
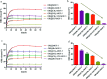
Metal organic frameworks (MOFs) are widely used in drug carrier research due to their tunability. The properties of MOFs can be adjusted through incorporation of mono-substituents to obtain pharmaceutical carriers with excellent properties. In this study, different functional groups of -NH2, -CH3, -Br, -OH and -CH2[double bond, length as m-dash]CH are connected to MOF-5 to analyse the effect of mono-substituent incorporation on drug delivery properties. The resulting MOFs have similar structures, except for Br-MOF. The pore size of this series of MOFs ranges from 1.04 nm to 1.10 nm. Using oridonin (ORI) as a model drug, introduction of the functional groups appears to have a significant effect on the drug delivery performance of the MOFs. The IRMOFs can be ranked according to drug-loading capacity: MOF-5 > HO-MOF-5 > H3C-MOF-5 = Br-MOF-5 > H2N-MOF-5 > CH2[double bond, length as m-dash]CH-MOF-5. The ORI release from ORI @IRMOFs is explored at two different pH values: 7.4 and 5.5, and the ORI@IRMOFs are ranked according to the cumulative release percentage of ORI: ORI@MOF-5 > ORI@Br-MOF-5 > ORI@H3C-MOF-5 > ORI@H2N-MOF-5 > CH2[double bond, length as m-dash]CH-MOF-5 > ORI@ HO-MOF-5. In particular, the release behaviour of ORI@MOFs is described through a new model. The different drug delivery performance of MOFs may be due to the complex interactions between MOFs and ORI. In addition, the introduction of single substituents does not change the biocompatibility of MOFs. MTT in vitro experiments prove that this series of MOFs has low cytotoxicity. This study shows that the incorporation of single substituents can effectively adjust the drug delivery behaviour of MOFs, which is conducive to realization of personalized drug delivery modes. The introduction of active groups can also facilitate post-synthesis modification to achieve coupling of targeting groups. MOFs incorporated with single substituents perform favorably in terms of use as biomedical drug delivery alternative carriers in effective drug payload and flexible drug release.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Aperture Modulation of Isoreticular Metal Organic Frameworks for Targeted Antitumor Drug Delivery.ACS Appl Mater Interfaces. 2022 Aug 17;14(32):36366-36378. doi: 10.1021/acsami.2c07450. Epub 2022 Jul 27. ACS Appl Mater Interfaces. 2022. PMID: 35897121
-
Investigation of Metal-Organic Framework-5 (MOF-5) as an Antitumor Drug Oridonin Sustained Release Carrier.Molecules. 2019 Sep 16;24(18):3369. doi: 10.3390/molecules24183369. Molecules. 2019. PMID: 31527488 Free PMC article.
-
Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers.Molecules. 2018 Sep 28;23(10):2490. doi: 10.3390/molecules23102490. Molecules. 2018. PMID: 30274195 Free PMC article.
-
Recent Advances in Metal-Organic Frameworks as Anticancer Drug Delivery Systems: A Review.Anticancer Agents Med Chem. 2021;21(18):2487-2504. doi: 10.2174/1871520621666210119093844. Anticancer Agents Med Chem. 2021. PMID: 33463479 Review.
-
Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer.Pharmaceutics. 2020 Mar 5;12(3):232. doi: 10.3390/pharmaceutics12030232. Pharmaceutics. 2020. PMID: 32151012 Free PMC article. Review.
Cited by
-
MOFs for next-generation cancer therapeutics through a biophysical approach-a review.Front Bioeng Biotechnol. 2024 Jun 13;12:1397804. doi: 10.3389/fbioe.2024.1397804. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38938982 Free PMC article. Review.
-
Niosomes as a Promising Therapeutic Approach against Colorectal Cancer: A Focus on the Delivery of Chemotherapeutics and Natural Products.Curr Pharm Des. 2024;30(21):1659-1666. doi: 10.2174/0113816128303645240429052835. Curr Pharm Des. 2024. PMID: 38747232 Review.
-
Novel UiO-NH2-like Zr-Based MOF (Basu-DPU) as an Excellent Catalyst for Preparation of New 6H-Chromeno[4,3-b]quinolin-6-ones.ACS Omega. 2023 Jul 12;8(29):25924-25937. doi: 10.1021/acsomega.3c01793. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521649 Free PMC article.
-
Negatively Charged MOF-Based Composite Anion Exchange Membrane with High Cation Selectivity and Permeability.Membranes (Basel). 2022 Jun 10;12(6):601. doi: 10.3390/membranes12060601. Membranes (Basel). 2022. PMID: 35736308 Free PMC article.
-
Microwave-Responsive Metal-Organic Frameworks (MOFs) for Enhanced In Vitro Controlled Release of Doxorubicin.Nanomaterials (Basel). 2024 Jun 24;14(13):1081. doi: 10.3390/nano14131081. Nanomaterials (Basel). 2024. PMID: 38998686 Free PMC article.
References
LinkOut - more resources
Full Text Sources

