Advances in chemistry and composition of soft materials for drug releasing contact lenses
- PMID: 35517957
- PMCID: PMC9057048
- DOI: 10.1039/d0ra06681h
Advances in chemistry and composition of soft materials for drug releasing contact lenses
Abstract
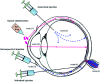
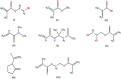
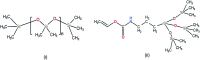
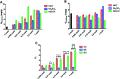
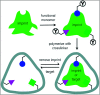
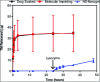
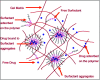
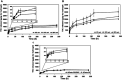
Ocular drug delivery has always been a challenging feat to achieve in the field of medical sciences. One of the existing methods of non-invasive ocular drug delivery is the use of eye drops. However, drugs administered through these formulations have low bioavailability in the ocular system. This limitation can been overcome by using contact lenses as drug delivery vehicles. According to USA FDA definitions they can be categorized into two main categories-hard and soft contact lenses. Based on the material properties, hard contact lenses are mostly produced from polymers of acrylate monomers such as MMA (methyl methacrylate). These have the least water retention capacity, thereby, having minimal ability to diffuse oxygen into the corneal layer and are not ideal for long term use. Soft material contact lenses are flexible and are mainly hydrogel based. They have higher water retention capacities as compared to rigid contact lenses, which gives them the ability to transmit oxygen to the corneal layer. These hydrogel based soft materials are mainly produced from polymers of acrylate monomers such as HEMA (hydroxyethyl methacrylate) and found to be better for drug delivery contact lenses. These polymer-based soft materials have been efficiently modified in terms of their chemistry to achieve diverse physicochemical properties to produce efficient ocular drug delivery systems. However, complications such as drug leaching during storage and distribution, sterilisation, preservation of integrity of the lens and the possibility of surface roughness due to the incorporated drug molecules still need to be optimised. This review highlights the chemistries of various polymeric molecules through which physicochemical properties can be modified to achieve optimum drug loading and sustained release of the drug for application in the ocular system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Rowe-Rendleman C. L. Durazo S. A. Kompella U. B. Rittenhouse K. D. Di Polo A. Weiner A. L. Grossniklaus H. E. Naash M. I. Lewin A. S. Horsager A. Edelhauser H. F. Drug and gene delivery to the back of the eye: from bench to bedside. Investig. Ophthalmol. Vis. Sci. 2014;55(4):2714–2730. doi: 10.1167/iovs.13-13707. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

