Artificial sugar saccharin and its derivatives: role as a catalyst
- PMID: 35517977
- PMCID: PMC9057081
- DOI: 10.1039/d0ra05974a
Artificial sugar saccharin and its derivatives: role as a catalyst
Abstract
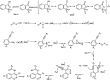
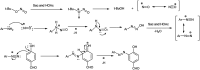
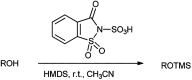
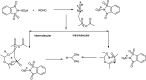
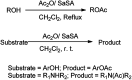
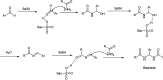
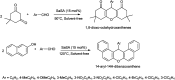
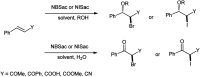
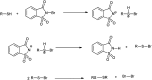
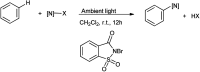
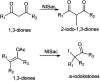
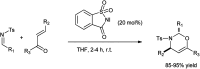
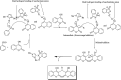
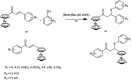
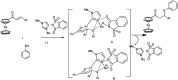
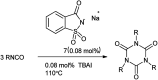
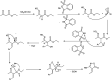
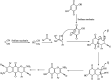
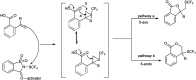
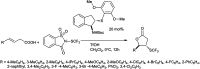
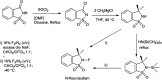
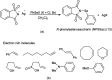
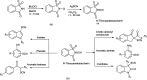
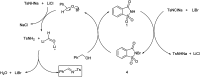
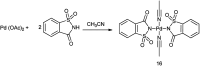
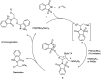
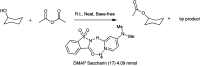
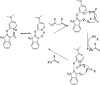
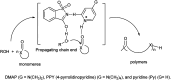
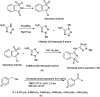
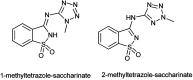
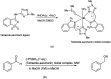
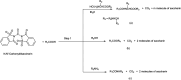
The primary objective of this review was to demonstrate the significance of artificial sugar saccharin and its derivatives as catalysts for a wide variety of organic transformations. The application of saccharin and its derivatives represents a greener and superior catalytic approach for reactions. In particular, we were interested in bringing together the literature pertaining to these saccharin derivatives from a catalysis perspective. The present review reports synthesis of saccharin and its derivatives such as saccharin-N-sulfonic acid, sodium saccharin, N-halo saccharin, saccharin lithium-bromide, N-formyl saccharin, N-acyl saccharin, N-nitrosaccharin, N-SCF3 saccharin, N-fluorosultam, N-phenylselenosaccharin, N-thiocyanatosaccharin palladium saccharin, DMAP-saccharin, and [Bmim]Sac. This catalytic application of saccharin and its derivatives includes reactions such as the Biginelli reaction, Paal-Knorr pyrrole synthesis, azo-coupling reaction, halogenations, domino Knoevenagel, Michael, deoximation reaction, catalytic condensation, functional group protection and oxidation etc. Also, these saccharin derivatives act as a source of CO, NH2, SCN, SCF3 and nitro groups. We reported all the available data on saccharin and its derivatives acting as a catalyst from 1957 to date.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflict to declare.
Figures
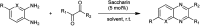
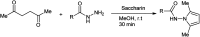
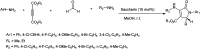
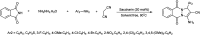
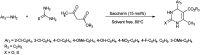

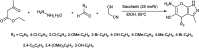
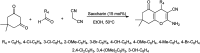
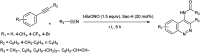
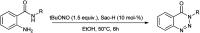
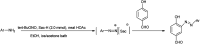
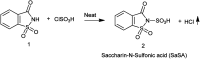
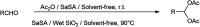
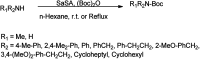
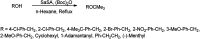
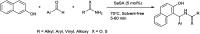
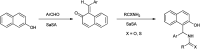
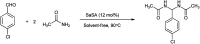
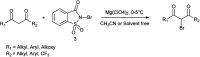
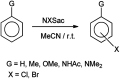
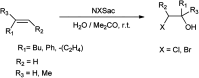
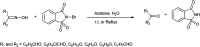
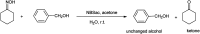
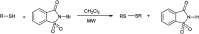
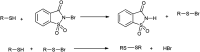
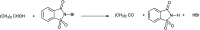
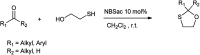

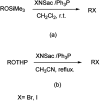
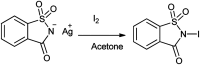
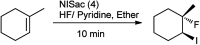
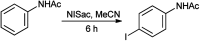
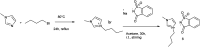
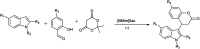
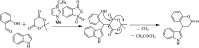
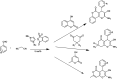
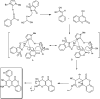
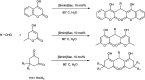

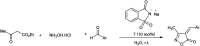
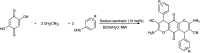
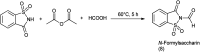
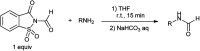
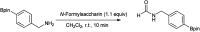
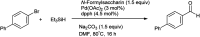
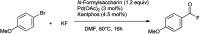
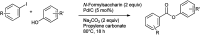
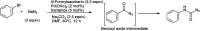
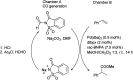


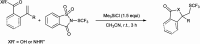
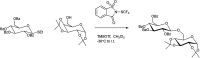
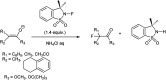
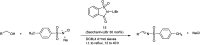

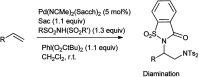
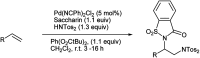


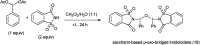
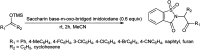
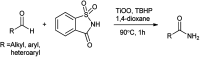
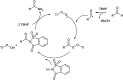
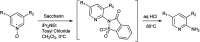
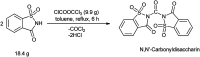


References
-
- Kumar A. Tripathi V. D. Kumar P. Green Chem. 2011;13:51–54. doi: 10.1039/C0GC00523A. - DOI
-
- Dhartiben B. K. Aparnathi K. D. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(6):1283–1296. doi: 10.20546/ijcmas.2017.606.151. - DOI
-
- Neltner T. G. Kulkami N. R. Alger H. M. Maffini M. V. Bongard E. D. Fortin N. D. Olson E. D. Compr. Rev. Food Sci. Food Saf. 2011;10(6):342–368. doi: 10.1111/j.1541-4337.2011.00166.x. - DOI
-
- Srivastava S. New J. Chem. 2019;43:6469–6471. doi: 10.1039/C9NJ00538B. - DOI
- Kumar A. Srivastava S. Gupta G. Green Chem. 2012;14:3269–3272. doi: 10.1039/C2GC36276G. - DOI
- Kumar A. Srivastava S. Gupta G. Chaturvedi V. Sinha S. Srivastava R. ACS Comb. Sci. 2011;13:65–71. doi: 10.1021/co100022h. - DOI - PubMed
- Kumar A. Srivastava S. Gupta G. Tetrahedron Lett. 2010;51:517–520. doi: 10.1016/j.tetlet.2009.11.057. - DOI
- Kumar A. Gupta G. Srivastava S. J. Comb. Chem. 2010;12:458–462. doi: 10.1021/cc100007a. - DOI - PubMed
- Srivastava S. ChemistrySelect. 2020;5(2):799–803. doi: 10.1002/slct.201904806. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

