Preparation of a novel fracturing fluid with good heat and shear resistance
- PMID: 35518047
- PMCID: PMC9059618
- DOI: 10.1039/c8ra09483g
Preparation of a novel fracturing fluid with good heat and shear resistance
Abstract
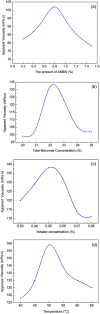
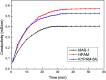
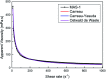
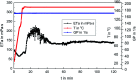
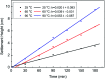
A new terpolymer (MAS-1) was created by copolymerizing acrylamide (AM), acrylic acid (AA), and 4-isopropenylcarbamoyl-benzene sulfonic acid (AMBS). Thermogravimetric analysis (TGA) suggested that MAS-1 has better heat resistance and meets the requirements for a fracturing fluid at 200 °C. X-ray diffraction (XRD), and conductivity tests showed that MAS-1 has good water solubility. The viscosity of the fracturing fluids containing MAS-1 was found to be about 135 mPa s after 120 min at 170 s-1 and 150 °C. SEM images and the determination of viscoelasticity showed that MAS-1 has a dense and robust spatial network structure in the fracturing fluid. The sedimentation velocity of the proppant was 0.0528 cm min-1 at 90 °C. When enough ammonium persulfate was added to yield approximately 0.10 wt%, the viscosity of the broken fluid was 4.5 mPa s, and the gel broken fluid was transparent without visible residue. In addition, the fracturing fluid did little damage to the reservoir. The drag reduction rate of MAS-1 was always higher than KYPAM-6A and HPAM with the shear rate ranging from 1000 s-1 to 7000 s-1. Therefore, this fracturing fluid could be an alternative for low permeability reservoir stimulation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
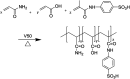










References
-
- Sun B. Wang J. Wang Z. Gao Y. Xu J. J. Pet. Sci. Eng. 2018;166:420–432. doi: 10.1016/j.petrol.2018.03.051. - DOI
-
- Wang J. Yin R. Liu Q. Fan Z. Adv. Pet. Explor. Dev. 2016;10:140–146.
-
- Zeng Q. Yao J. Computation. 2015;3:541–557. doi: 10.3390/computation3040541. - DOI
-
- Ihejirika B., Dosunmu A. and Eme C., Performance Evaluation of Guar Gum as a Carrier Fluid for Hydraulic Fracturing, Society of Petroleum Engineers, 2015
-
- Kekacs D., Treatment and Characterization of Hydraulic Fracturing Fluids, The Ohio State University, 2014
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

