Preparation of novel 1,2,3-triazole furocoumarin derivatives via click chemistry and their anti-vitiligo activity
- PMID: 35518056
- PMCID: PMC9059643
- DOI: 10.1039/c8ra09755k
Preparation of novel 1,2,3-triazole furocoumarin derivatives via click chemistry and their anti-vitiligo activity
Abstract
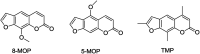
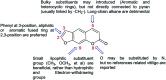
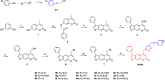
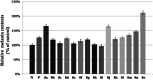
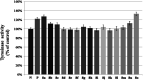
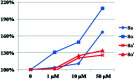
The extracts of Psoralea corylifolia L. were often used for the repigmentation of leukoderma (vitiligo) in traditional Uygur medicine thousands years ago. Nowadays, its active ingredient, furocoumarins, has been clinically applied since it exhibited strong photosensitivity. Thus, a new series of furocoumarin derivatives (8a-8o) containing 1,2,3-triazole were designed and synthesized based on our previous work. After biological evaluation for melanin contents and tyrosinase activity in B16 murine cells, the SAR was summarized. The results indicated that five compounds (8a, 8j, 8m-8o) were more potent than the positive control (8-MOP) on melanogenesis. Among them, 8a and 8o showed the best stimulating effect on tyrosinase activity as well, and were submitted for further pharmacological study of anti-vitiligo.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Novel Furocoumarin Derivatives Stimulate Melanogenesis in B16 Melanoma Cells by Up-Regulation of MITF and TYR Family via Akt/GSK3β/β-Catenin Signaling Pathways.Int J Mol Sci. 2018 Mar 6;19(3):746. doi: 10.3390/ijms19030746. Int J Mol Sci. 2018. PMID: 29509689 Free PMC article.
-
Study of Novel Furocoumarin Derivatives on Anti-Vitiligo Activity, Molecular Docking and Mechanism of Action.Int J Mol Sci. 2022 Jul 19;23(14):7959. doi: 10.3390/ijms23147959. Int J Mol Sci. 2022. PMID: 35887323 Free PMC article.
-
Synthesis and biological evaluation of furocoumarin derivatives on melanin synthesis in murine B16 cells for the treatment of vitiligo.Bioorg Med Chem. 2016 Nov 15;24(22):5960-5968. doi: 10.1016/j.bmc.2016.09.056. Epub 2016 Sep 23. Bioorg Med Chem. 2016. PMID: 27713014
-
Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo.Molecules. 2017 Aug 4;22(8):1303. doi: 10.3390/molecules22081303. Molecules. 2017. PMID: 28777326 Free PMC article. Review.
-
The Chemical Constituents and Bioactivities of Psoralea corylifolia Linn.: A Review.Am J Chin Med. 2016;44(1):35-60. doi: 10.1142/S0192415X16500038. Am J Chin Med. 2016. PMID: 26916913 Review.
Cited by
-
Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects.Int J Mol Sci. 2020 Aug 6;21(16):5622. doi: 10.3390/ijms21165622. Int J Mol Sci. 2020. PMID: 32781533 Free PMC article. Review.
-
Bakuchiol, a natural constituent and its pharmacological benefits.F1000Res. 2023 Nov 7;12:29. doi: 10.12688/f1000research.129072.2. eCollection 2023. F1000Res. 2023. PMID: 38021404 Free PMC article. Review.
-
An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy.Biomedicines. 2023 Jan 16;11(1):224. doi: 10.3390/biomedicines11010224. Biomedicines. 2023. PMID: 36672732 Free PMC article. Review.
References
-
- Ezzedine K. Lim H. W. Suzuki T. Katayama I. Hamzavi I. Lan C. C. E. Goh B. K. Anbar T. Silva de Castro C. Lee A. Y. Parsad D. van Geel N. Le Poole I. C. Oiso N. Benzekri L. Spritz R. Gauthier Y. Hann S. K. Picardo M. Taieb A. Pigm. Cell Melanoma Res. 2012;25:E1–E13. doi: 10.1111/j.1755-148X.2012.00997.x. - DOI - PMC - PubMed
-
- Wang S. Jin R. Wang R. Hu Y. Dong X. Xu A. RSC Adv. 2016;6:106308–106315. doi: 10.1039/C6RA23172A. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

