Efficient synthesis of C15 fuel precursor by heterogeneously catalyzed aldol-condensation of furfural with cyclopentanone
- PMID: 35518068
- PMCID: PMC9060462
- DOI: 10.1039/c8ra09517e
Efficient synthesis of C15 fuel precursor by heterogeneously catalyzed aldol-condensation of furfural with cyclopentanone
Abstract
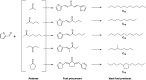
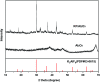
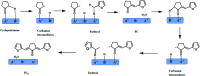
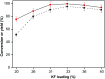
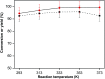
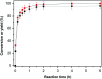
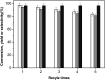
The aldol-condensation reaction of biomass-derived furfural and cyclopentanone coupled with hydrogenation/hydrodeoxygenation is a promising route for the production of renewable high-quality diesel or jet fuel. In this paper, we focus on the heterogeneously catalyzed aldol condensation of furfural with cyclopentanone, in order to maximize the yield of 2,5-bis(2-furylmethylidene)cyclopentan-1-one (F2C, a C15 fuel precursor). Experiments were conducted over a series of solid-base catalysts and it was found that potassium fluoride impregnated alumina shown the highest catalytic efficiency. We further investigated the effect of different parameters on the performance of KF/γ-Al2O3, including KF loading amount, solvent, reaction time and temperature. Over KF/γ-Al2O3 with a 33% KF loading amount, the aldol reaction achieved an F2C molar yield up to 95.4% at 333 K within 2 h. Under the nearly optimal condition, the separated solid product is almost F2C with a purity of 100%, providing a high-quality biofuel precursor for the next processing step.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF.Molecules. 2023 Jul 14;28(14):5397. doi: 10.3390/molecules28145397. Molecules. 2023. PMID: 37513268 Free PMC article. Review.
-
Enhanced production of jet fuel precursors via furfural/cyclopentanone aldol condensation by synergistic pairing TiO2 with nano-ZSM-5 zeolite.Bioresour Technol. 2025 Feb;418:131877. doi: 10.1016/j.biortech.2024.131877. Epub 2024 Nov 26. Bioresour Technol. 2025. PMID: 39608420
-
Production of renewable long-chained cycloalkanes from biomass-derived furfurals and cyclic ketones.RSC Adv. 2018 Apr 12;8(25):13686-13696. doi: 10.1039/c8ra01723a. eCollection 2018 Apr 11. RSC Adv. 2018. PMID: 35539315 Free PMC article.
-
Synthesis of Renewable High-Density Fuel with Vanillin and Cyclopentanone Derived from Hemicellulose.Molecules. 2023 Jun 27;28(13):5029. doi: 10.3390/molecules28135029. Molecules. 2023. PMID: 37446694 Free PMC article.
-
Research progress of catalysts for aldol condensation of biomass based compounds.RSC Adv. 2023 Mar 23;13(14):9466-9478. doi: 10.1039/d3ra00906h. eCollection 2023 Mar 20. RSC Adv. 2023. PMID: 36968059 Free PMC article. Review.
Cited by
-
Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review.ACS Omega. 2021 Dec 15;6(51):35145-35172. doi: 10.1021/acsomega.1c05861. eCollection 2021 Dec 28. ACS Omega. 2021. PMID: 34984249 Free PMC article. Review.
-
Ambient-pressure lignin valorization to high-performance polymers by intensified reductive catalytic deconstruction.Sci Adv. 2022 Jan 21;8(3):eabj7523. doi: 10.1126/sciadv.abj7523. Epub 2022 Jan 19. Sci Adv. 2022. PMID: 35044829 Free PMC article.
-
Intensifying Cyclopentanone Synthesis from Furfural Using Supported Copper Catalysts.ChemSusChem. 2025 Feb 16;18(4):e202401484. doi: 10.1002/cssc.202401484. Epub 2024 Nov 8. ChemSusChem. 2025. PMID: 39340337 Free PMC article.
-
A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF.Molecules. 2023 Jul 14;28(14):5397. doi: 10.3390/molecules28145397. Molecules. 2023. PMID: 37513268 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

