Study of the perpendicular self-assembly of a novel high- χ block copolymer without any neutral layer on a silicon substrate
- PMID: 35518108
- PMCID: PMC9060441
- DOI: 10.1039/c8ra10319d
Study of the perpendicular self-assembly of a novel high- χ block copolymer without any neutral layer on a silicon substrate
Abstract
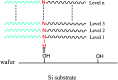
A novel type of high-χ block copolymer, polystyrene-block-polycarbonate (PS-b-PC), which contains an active -NH- group on the polymer backbone between the PS block and the PC block, has been successfully synthesized. Vertical micro-phase separation can be successfully achieved on Si substrates with neutral-layer-free materials with a pitch of 16.8 nm. Water contact angle experiments indicate that PS and PC have approximate surface energy values on Si substrates. A hydrogen bond mechanism has been proposed for the formation of a periodic and lamella-forming phase separation structure, with the domains oriented perpendicular to the substrate. A combination of both theory and experimental verification proves that the hydrogen bonding plays a dominant role as a real driving force to promote vertical micro-phase separation in the absence of a neutral layer. Subsequently, the study of a novel block copolymer on four different types of substrate without any neutral layer further confirms that the newly synthesized material enables greater flexibility and potential applications for the fabrication of various nanostructures and functional electronic devices in a simple, cost-effective and efficient way, which is of considerable importance to contemporary and emerging technology applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








Similar articles
-
Focused solar annealing for block copolymer fast self-assembly.Heliyon. 2024 Jan 5;10(2):e24016. doi: 10.1016/j.heliyon.2024.e24016. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293481 Free PMC article.
-
Phosphorene-directed self-assembly of asymmetric PS-b-PMMA block copolymer for perpendicularly-oriented sub-10 nm PS nanopore arrays.Nanotechnology. 2017 Oct 20;28(42):424001. doi: 10.1088/1361-6528/aa8748. Epub 2017 Aug 21. Nanotechnology. 2017. PMID: 28825413
-
Orientation Control in Thin Films of a High-χ Block Copolymer with a Surface Active Embedded Neutral Layer.Nano Lett. 2016 Jan 13;16(1):728-35. doi: 10.1021/acs.nanolett.5b04602. Epub 2015 Dec 23. Nano Lett. 2016. PMID: 26682931
-
Perpendicular Orientation Control without Interfacial Treatment of RAFT-Synthesized High-χ Block Copolymer Thin Films with Sub-10 nm Features Prepared via Thermal Annealing.ACS Appl Mater Interfaces. 2017 Sep 20;9(37):31266-31278. doi: 10.1021/acsami.6b16129. Epub 2017 Mar 17. ACS Appl Mater Interfaces. 2017. PMID: 28304153
-
Orientation-controlled self-assembled nanolithography using a polystyrene-polydimethylsiloxane block copolymer.Nano Lett. 2007 Jul;7(7):2046-50. doi: 10.1021/nl070924l. Epub 2007 Jun 15. Nano Lett. 2007. PMID: 17570733 Review.
Cited by
-
An experimental and theoretical investigation into the self-assembly of a chemically modified high-χ coil-rod diblock copolymer.RSC Adv. 2022 Jun 17;12(28):17950-17958. doi: 10.1039/d2ra02536a. eCollection 2022 Jun 14. RSC Adv. 2022. PMID: 35765337 Free PMC article.
References
LinkOut - more resources
Full Text Sources

