Development and application of novel bio-magnetic membrane capsules for the removal of the cationic dye malachite green in wastewater treatment
- PMID: 35518114
- PMCID: PMC9060252
- DOI: 10.1039/c8ra09275c
Development and application of novel bio-magnetic membrane capsules for the removal of the cationic dye malachite green in wastewater treatment
Abstract
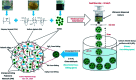
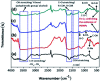
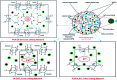
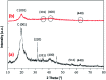
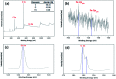
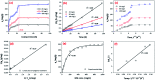
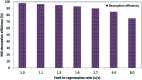
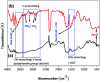
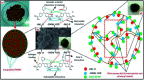
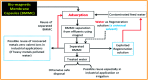
Novel bio-magnetic membrane capsules (BMMCs) were prepared by a simple two-step titration-gel cross-linking method using a polyvinyl alcohol (PVA) and sodium alginate (SA) matrix to control the disintegration of phytogenic magnetic nanoparticles (PMNPs) in an aqueous environment, and their performance was investigated for adsorbing cationic malachite green (MG) dye from water. The prepared BMMCs were characterized by FTIR, powder XRD, SEM, EDX, XPS, VSM and TGA techniques. The findings revealed that the hysteresis loops had an excellent superparamagnetic nature with saturation magnetization values of 11.02 emu g-1. The prepared BMMCs not only controlled the oxidation of PMNPs but also improved the adsorptive performance with respect to MG dye (500 mg g-1 at 298.15 K and pH 6.5) due to the presence of a large amount of hydrophilic functional groups (hydroxyl/-OH and carboxyl/-COOH) on/in the BMMCs. The smooth encapsulation of PMNPs into the PVA-SA matrix established additional hydrogen bonding among polymer molecular chains, with improved stability, and adsorptive performance was maintained over a wide range of pH values (3-12). Importantly, the prepared BMMCs were easily regenerated just by washing with water, and they could be re-utilized for up to four (4) consecutive treatment cycles without observing any apparent dissolution of iron/Fe0 or damage to the morphology. According to the mass balance approach, an estimated amount of 100 mL of treated effluent can be obtained from 160 mL of MG dye solution (25 mg L-1) just by employing a 0.02 g L-1 adsorbent dosage. Finally, a model of BMMCs based on zero-effluent discharge was also proposed for commercial or industrial applications. The prepared BMMCs are greatly needed for improving the water/wastewater treatment process and they can be utilized as an excellent adsorbent to remove cationic pollutants for various environmental applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Encapsulated green magnetic nanoparticles for the removal of toxic Pb2+ and Cd2+ from water: Development, characterization and application.J Environ Manage. 2019 Mar 15;234:273-289. doi: 10.1016/j.jenvman.2018.12.112. Epub 2019 Jan 8. J Environ Manage. 2019. PMID: 30634120
-
Sorption of cationic malachite green dye on phytogenic magnetic nanoparticles functionalized by 3-marcaptopropanic acid.RSC Adv. 2018 Feb 27;8(16):8878-8897. doi: 10.1039/c8ra00245b. eCollection 2018 Feb 23. RSC Adv. 2018. PMID: 35539840 Free PMC article.
-
Development of a novel three-dimensional magnetic polymer aerogel as an efficient adsorbent for malachite green removal.J Hazard Mater. 2020 Feb 15;384:121394. doi: 10.1016/j.jhazmat.2019.121394. Epub 2019 Oct 6. J Hazard Mater. 2020. PMID: 31628059
-
Turning calcium carbonate into a cost-effective wastewater-sorbing material by occluding waste dye.Environ Sci Pollut Res Int. 2010 Jan;17(1):97-105. doi: 10.1007/s11356-009-0111-y. Epub 2009 Mar 5. Environ Sci Pollut Res Int. 2010. PMID: 19263103
-
Synthesis of Fe3O4@chitosan@ZIF-8 towards removal of malachite green from aqueous solution: Theoretical and experimental studies.Int J Biol Macromol. 2021 Jan 31;168:428-441. doi: 10.1016/j.ijbiomac.2020.12.067. Epub 2020 Dec 10. Int J Biol Macromol. 2021. PMID: 33310100
Cited by
-
The Evaluation of Cellulose Acetate Capsules Functionalized for the Removal of Cd(II).Polymers (Basel). 2023 Sep 28;15(19):3917. doi: 10.3390/polym15193917. Polymers (Basel). 2023. PMID: 37835966 Free PMC article.
-
Sunlight-assisted degradation of textile pollutants and phytotoxicity evaluation using mesoporous ZnO/g-C3N4 catalyst.RSC Adv. 2021 Aug 5;11(43):26800-26812. doi: 10.1039/d1ra03806k. eCollection 2021 Aug 2. RSC Adv. 2021. PMID: 35480009 Free PMC article.
-
An Updated Overview of Magnetic Composites for Water Decontamination.Polymers (Basel). 2024 Mar 5;16(5):709. doi: 10.3390/polym16050709. Polymers (Basel). 2024. PMID: 38475395 Free PMC article. Review.
-
Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers.Polymers (Basel). 2020 Sep 2;12(9):1999. doi: 10.3390/polym12091999. Polymers (Basel). 2020. PMID: 32887444 Free PMC article. Review.
-
Construction of a phosphate-rich polyacrylonitrile fiber surface microenvironment for efficient purification of crystal violet wastewater.RSC Adv. 2019 Nov 19;9(64):37630-37641. doi: 10.1039/c9ra07199g. eCollection 2019 Nov 13. RSC Adv. 2019. PMID: 35542276 Free PMC article.
References
-
- Ali I. Peng C. Naz I. Khan Z. M. Sultan M. Islama T. Abbasi I. A. RSC Adv. 2017a;7:40158–40178.
-
- Bekin S. Sarmad S. Gürkan K. Keçeli G. Gürdağ G. Sens. Actuators, B. 2014;202:878–892. doi: 10.1016/j.snb.2014.06.051. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

