In vitro and in vivo biocompatibility and inflammation response of methacrylated and maleated hyaluronic acid for wound healing
- PMID: 35518130
- PMCID: PMC9056621
- DOI: 10.1039/d0ra06025a
In vitro and in vivo biocompatibility and inflammation response of methacrylated and maleated hyaluronic acid for wound healing
Abstract
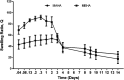
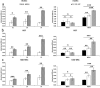
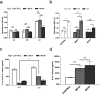
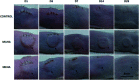
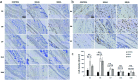
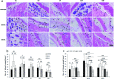
Over the past few years, different in vitro and in vivo studies have been highlighting the great potentiality of hyaluronic acid (HA) as a biomaterial in wound healing treatment thanks to its good capability to induce mesenchymal and epithelial cell growth and differentiation, angiogenesis, and collagen deposition. However, the need to improve its mechanical properties as well as its residence time has led scientists to study new functionalization strategies. In this work, chemically modified HA-based hydrogels were obtained by methacrylic and maleic functionalization. Methacrylated (MEHA) and maleated HA (MAHA) hydrogels have shown important physico-chemical properties. The present study provides a deeper insight into the biocompatibility of both synthesized materials and their effects on tissue inflammation using in vitro and in vivo models. To this aim, different cell lines involved in wound healing, human dermal fibroblasts, human adipose-derived stem cells and human umbilical vein endothelial cells, were seeded on MEHA and MAHA hydrogels. Furthermore, an inflammation study was carried out on a murine macrophage cell line to assess the effects of both hydrogels on inflammatory and anti-inflammatory interleukin production. The results showed that both MAHA and MEHA supported cell proliferation with anti-inflammation ability as highlighted by the increased levels of IL-10 (57.92 ± 9.87 pg mL-1 and 68.08 ± 13.94 pg mL-1, for MEHA and MAHA, respectively). To investigate the inflammatory response at tissue/implant interfaces, an in vivo study was also performed by subcutaneous implantation of the materials in BALB/c mice for up to 28 days. In these analyses, no significant chronic inflammation reaction was demonstrated in either MEHA or MAHA in the long-term implantation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest related to this study.
Figures


Similar articles
-
Dynamic mechanical and swelling properties of maleated hyaluronic acid hydrogels.Carbohydr Polym. 2015 Jun 5;123:381-9. doi: 10.1016/j.carbpol.2015.01.047. Epub 2015 Feb 10. Carbohydr Polym. 2015. PMID: 25843871
-
Exosomes of mesenchymal stem cells delivered from methacrylated hyaluronic acid patch improve the regenerative properties of endothelial and dermal cells.Biomater Adv. 2022 Aug;139:213000. doi: 10.1016/j.bioadv.2022.213000. Epub 2022 Jun 25. Biomater Adv. 2022. PMID: 35891601
-
Bioactive composites based on double network approach with tailored mechanical, physico-chemical, and biological features.J Biomed Mater Res A. 2018 Dec;106(12):3079-3089. doi: 10.1002/jbm.a.36498. Epub 2018 Sep 12. J Biomed Mater Res A. 2018. PMID: 30208257
-
3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity.PLoS One. 2017 Jun 6;12(6):e0177628. doi: 10.1371/journal.pone.0177628. eCollection 2017. PLoS One. 2017. PMID: 28586346 Free PMC article.
-
Advances in modified hyaluronic acid-based hydrogels for skin wound healing.Biomater Sci. 2022 Jun 28;10(13):3393-3409. doi: 10.1039/d2bm00397j. Biomater Sci. 2022. PMID: 35575243 Review.
Cited by
-
Material matters: exploring the interplay between natural biomaterials and host immune system.Front Immunol. 2023 Oct 23;14:1269960. doi: 10.3389/fimmu.2023.1269960. eCollection 2023. Front Immunol. 2023. PMID: 37936689 Free PMC article. Review.
-
Advances in 3D Culture Models to Study Exosomes in Triple-Negative Breast Cancer.Cancers (Basel). 2024 Feb 22;16(5):883. doi: 10.3390/cancers16050883. Cancers (Basel). 2024. PMID: 38473244 Free PMC article. Review.
-
Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources.J Funct Biomater. 2022 Aug 12;13(3):118. doi: 10.3390/jfb13030118. J Funct Biomater. 2022. PMID: 35997456 Free PMC article. Review.
-
Fish Gelatin-Hyaluronic Acid Scaffold for Construction of an Artificial Three-Dimensional Skin Model.ACS Omega. 2025 Feb 21;10(8):8172-8181. doi: 10.1021/acsomega.4c09708. eCollection 2025 Mar 4. ACS Omega. 2025. PMID: 40060871 Free PMC article.
-
Advances in the Physico-Chemical, Antimicrobial and Angiogenic Properties of Graphene-Oxide/Cellulose Nanocomposites for Wound Healing.Pharmaceutics. 2023 Jan 19;15(2):338. doi: 10.3390/pharmaceutics15020338. Pharmaceutics. 2023. PMID: 36839660 Free PMC article. Review.
References
-
- Oommen O. P. Wang S. Kisiel M. Sloff M. Hilborn J. Varghese O. P. Adv. Funct. Mater. 2013;23:1273–1280. doi: 10.1002/adfm.201201698. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

