Boron Schiff bases derived from α-amino acids as nucleoli/cytoplasm cell-staining fluorescent probes in vitro
- PMID: 35518166
- PMCID: PMC9056538
- DOI: 10.1039/d0ra05948j
Boron Schiff bases derived from α-amino acids as nucleoli/cytoplasm cell-staining fluorescent probes in vitro
Abstract
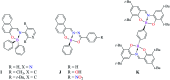
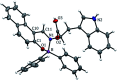
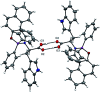
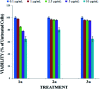
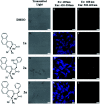
The size, shape, and number of nucleoli in a cell's nucleus might help to distinguish a malignant from a benign tumor. Cellular biology and histopathology often require better visualization to understand nucleoli-related processes, thus organelle-specific fluorescent markers are needed. Here, we report the design, synthesis, and fully chemo-photophysical characterization of fluorescent boron Schiff bases (BOSCHIBAs), derived from α-amino acids (i.e., phenylalanine, tyrosine and tryptophan), with nucleoli- and cytoplasm-specific staining in cells. It is the first time that Boron Schiff bases derived from α-amino acids act as notorious dual (nucleoli and cytoplasm) cell-staining fluorescent probes. The boron derivatives not only showed good photostability and acceptable quantum yields (∼5%) in solution, but also exhibited low cytotoxicity (>90% cell viability at 0.1 and 1 μg mL-1), which make them good candidates to be used in medical diagnosis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
LinkOut - more resources
Full Text Sources

