Heterodinuclear nickel(ii)-iron(ii) azadithiolates as structural and functional models for the active site of [NiFe]-hydrogenases
- PMID: 35518169
- PMCID: PMC9056516
- DOI: 10.1039/d0ra04344c
Heterodinuclear nickel(ii)-iron(ii) azadithiolates as structural and functional models for the active site of [NiFe]-hydrogenases
Abstract
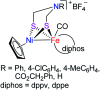
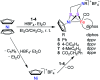
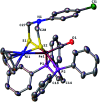
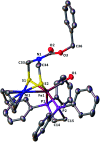
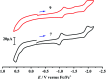
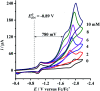
To develop the biomimetic chemistry of [NiFe]-H2ases, the first azadithiolato-bridged NiFe model complexes [CpNi{(μ-SCH2)2NR}Fe(CO)(diphos)]BF4 (5, R = Ph, diphos = dppv; 6, 4-ClC6H4, dppv; 7, 4-MeC6H4, dppv; 8, CO2CH2Ph, dppe; 9, H, dppe) have been synthesized via well-designed synthetic routes. Thus, treatment of RN[CH2S(O)CMe]2 with t-BuONa followed by reaction of the resulting intermediates RN(CH2SNa)2 with (dppv)Fe(CO)2Cl2 or (dppe)Fe(CO)2Cl2 gave the N-substituted azadithiolato-chelated Fe complexes [RN(CH2S)2]Fe(CO)2(diphos) (1, R = Ph, diphos = dppv; 2, 4-ClC6H4, dppv; 3, 4-MeC6H4, dppv; 4, CO2CH2Ph, dppe). Further treatment of 1-4 with nickelocene in the presence of HBF4·Et2O afforded the corresponding N-substituted azadithiolato-bridged NiFe model complexes 5-8, while treatment of 8 with HBF4·Et2O resulted in formation of the parent azadithiolato-bridged model complex 9. While all the new complexes 1-9 were characterized by elemental analysis and spectroscopy, the molecular structures of model complexes 6-8 were confirmed by X-ray crystallographic study. In addition, model complexes 7 and 9 were found to be catalysts for H2 production with moderate i cat/i p and overpotential values from TFA under CV conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


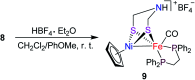


References
-
- Volbeda A. Garcin E. Piras C. de Lacey A. L. Fernandez V. M. Hatchikian E. C. Frey M. Fontecilla-Camps J. C. J. Am. Chem. Soc. 1996;118:12989–12996. doi: 10.1021/ja962270g. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

