Di-functional luminescent sensors based on Y3+ doped Eu3+ and Tb3+ coordination polymers: fast response and visible detection of Cr3+, Fe3+ ions in aqueous solutions and acetone
- PMID: 35518171
- PMCID: PMC9056567
- DOI: 10.1039/d0ra06407f
Di-functional luminescent sensors based on Y3+ doped Eu3+ and Tb3+ coordination polymers: fast response and visible detection of Cr3+, Fe3+ ions in aqueous solutions and acetone
Abstract
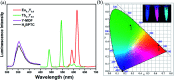
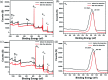
With the careful modulation of the relative ratio of Y3+/Eu3+and Y3+/Tb3+, two series of bimetallic RE-CPs (Eu x Y1- x and Tb x Y1- x ) were successfully obtained through the isomorphous substitution method. Interestingly, the introduction of Y3+ ions does not change the fluorescence characteristic peak of 1-Eu and 1-Tb, but enhances its fluorescence lifetime and quantum yield. Experimental and theoretical simulation results show the co-doping process changes the intramolecular energy transfer process and reduces the non-radiative transition resulting from concentration quenching. Eu0.1Y0.9 and Tb0.1Y0.9 with the largest luminescence lifetime were selected as the representative research objects, their potential application for the detection of toxic metal ions and organic molecules was further investigated. Interestingly, Eu0.1Y0.9 and Tb0.1Y0.9 demonstrate high sensitivity and good selectivity towards Fe3+, Cr3+ and acetone. Besides, fine fluorescence visibility provides the necessary conditions for the preparation of simple and fast response fluorescent test papers in order to achieve real-time and convenient detection of these toxic materials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









Similar articles
-
Water-Stable Carborane-Based Eu3+/Tb3+ Metal-Organic Frameworks for Tunable Time-Dependent Emission Color and Their Application in Anticounterfeiting Bar-Coding.Chem Mater. 2022 May 24;34(10):4795-4808. doi: 10.1021/acs.chemmater.2c00323. Epub 2022 Apr 29. Chem Mater. 2022. PMID: 35637791 Free PMC article.
-
Selective sensing of Fe3+ ions in aqueous solution by a biodegradable platform based lanthanide metal organic framework.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Apr 5;230:118084. doi: 10.1016/j.saa.2020.118084. Epub 2020 Jan 21. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 32000062
-
Effect of structure, particle size and relative concentration of Eu(3+) and Tb(3+) ions on the luminescence properties of Eu(3+) co-doped Y(2)O(3):Tb nanoparticles.Nanotechnology. 2008 Aug 13;19(32):325704. doi: 10.1088/0957-4484/19/32/325704. Epub 2008 Jul 4. Nanotechnology. 2008. PMID: 21828826
-
Fluorescent Eu3+/Tb3+ Metal-Organic Frameworks for Ratiometric Temperature Sensing Regulated by Ligand Energy.Inorg Chem. 2022 Sep 12;61(36):14322-14332. doi: 10.1021/acs.inorgchem.2c02025. Epub 2022 Aug 26. Inorg Chem. 2022. PMID: 36026489
-
Layered rare-earth hydroxide and oxide nanoplates of the Y/Tb/Eu system: phase-controlled processing, structure characterization and color-tunable photoluminescence via selective excitation and efficient energy transfer.Sci Technol Adv Mater. 2013 Feb 21;14(1):015006. doi: 10.1088/1468-6996/14/1/015006. eCollection 2013 Feb. Sci Technol Adv Mater. 2013. PMID: 27877564 Free PMC article. Review.
Cited by
-
Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone.Molecules. 2021 Mar 18;26(6):1695. doi: 10.3390/molecules26061695. Molecules. 2021. PMID: 33803563 Free PMC article.
References
-
- Singha D. K. Majee P. Mondal S. K. Mahata P. RSC Adv. 2015;5:102076–102084. doi: 10.1039/C5RA22599J. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

