Characterization of insulin cross-seeding: the underlying mechanism reveals seeding and denaturant-induced insulin fibrillation proceeds through structurally similar intermediates
- PMID: 35518209
- PMCID: PMC9056291
- DOI: 10.1039/d0ra05414c
Characterization of insulin cross-seeding: the underlying mechanism reveals seeding and denaturant-induced insulin fibrillation proceeds through structurally similar intermediates
Abstract
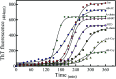
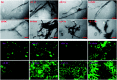
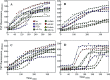
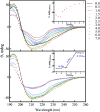
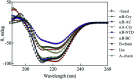
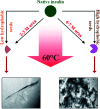
Insulin rapidly fibrillates in the presence of amyloid seeds from different sources. To address its cross-reactivity we chose the seeds of seven model proteins and peptides along with the seeds of insulin itself. Model candidates were selected/designed according to their size, amino acid sequence, and hydrophobicity. We found while some seeds provided catalytic ends for inducing the formation of non-native insulin conformers and increase fibrillation, others attenuated insulin fibrillation kinetics. We also observed competition between the intermediate insulin conformers which formed with urea and amyloid seeds in entering the fibrillogenic pathway. Simultaneous incubation of insulin with urea and amyloid seeds resulted in the formation of nearly similar insulin intermediate conformers which synergistically enhance insulin fibrillation kinetics. Given these results, it is highly likely that, structurally, there is a specific intermediate in different pathways of insulin fibrillation that governs fibrillation kinetics and morphology of the final mature fibril. Overall, this study provides a novel mechanistic insight into insulin fibrillation and gives new information on how seeds of different proteins are capable of altering insulin fibrillation kinetics and morphology. This report, for the first time, tries to answer an important question that why fibrillation of insulin is either accelerated or attenuated in the presence of amyloid fibril seeds from different sources.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism.Biochemistry. 2001 May 22;40(20):6036-46. doi: 10.1021/bi002555c. Biochemistry. 2001. PMID: 11352739
-
Independent heterologous fibrillation of insulin and its B-chain peptide.Biochemistry. 2005 Dec 20;44(50):16701-9. doi: 10.1021/bi051658y. Biochemistry. 2005. PMID: 16342960
-
Master and slave relationship between two types of self-propagating insulin amyloid fibrils.J Phys Chem B. 2014 Nov 26;118(47):13582-9. doi: 10.1021/jp510980b. Epub 2014 Nov 17. J Phys Chem B. 2014. PMID: 25373010
-
Formation mechanism of insulin fibrils and structural aspects of the insulin fibrillation process.Curr Protein Pept Sci. 2009 Oct;10(5):509-28. doi: 10.2174/138920309789352038. Curr Protein Pept Sci. 2009. PMID: 19538143 Review.
-
Amyloid fibril formation by human stefin B: influence of the initial pH-induced intermediate state.Biochem Soc Trans. 2002 Aug;30(4):543-7. doi: 10.1042/bst0300543. Biochem Soc Trans. 2002. PMID: 12196133 Review.
Cited by
-
Experimental methods for studying amyloid cross-interactions.Protein Sci. 2025 Jun;34(6):e70151. doi: 10.1002/pro.70151. Protein Sci. 2025. PMID: 40384558 Free PMC article. Review.
-
Inhibitory effect of coumarin and its analogs on insulin fibrillation /cytotoxicity is depend on oligomerization states of the protein.RSC Adv. 2020 Oct 16;10(63):38260-38274. doi: 10.1039/d0ra07710k. eCollection 2020 Oct 15. RSC Adv. 2020. PMID: 35517555 Free PMC article.
References
LinkOut - more resources
Full Text Sources

