Revisiting the photochemical synthesis of [FeFe]-hydrogenase mimics: reaction optimization, mechanistic study and electrochemical behaviour
- PMID: 35518225
- PMCID: PMC9056276
- DOI: 10.1039/d0ra06002j
Revisiting the photochemical synthesis of [FeFe]-hydrogenase mimics: reaction optimization, mechanistic study and electrochemical behaviour
Abstract
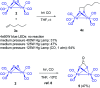
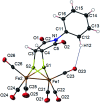
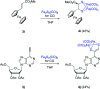
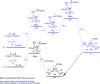
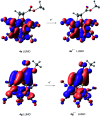
The photoreaction of [(μ-S)2Fe2(CO)6] and alkenes or alkynes has been optimized to readily obtain functionalized [FeFe]-hydrogenase mimics. Irradiation under low CO pressure in THF produces the corresponding photo-adducts in good/acceptable (alkenes/alkynes) yields, with retention of the starting olefin stereochemistry. DFT-calculations provide plausible reaction pathways in both, singlet and triplet states. The DFT-calculation based in the singlet state is energetically more favorable. The electrochemical behavior of the synthesized compounds is also presented, including studies in acidic media. The electrochemical properties of the products vary in the presence of a double bond (cycloaddition of [(μ-S)2Fe2(CO)6] to alkynes), respect to a single bond (cycloaddition to alkenes).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
- Hydrogen as a Fuel: Learning from Nature, ed. R. Cammack, M. Frey and R. Robson, Taylor&Francis, 2001
- Compendium of Hydrogen Energy Volume 1: Hydrogen Production and Purification, ed. V. Subramani, A. Basile and T. N. Veziroğlu, Elsevier, 2015
- Dincer I. and Zamfirescu C., Sustainable Hydrogen Production, Elsevier, 2016
- Compendium of Hydrogen Energy Volume 4: Hydrogen Use, Safety and the Hydrogen Economy, ed. M. Ball, A. Basile and T. N. Veziroğlu, Elsevier, 2016
- Corredor J. Rivero M. J. Rangel C. M. Gloaguen F. Ortiz I. J. Chem. Technol. Biotechnol. 2019;94:3049–3063. doi: 10.1002/jctb.6123. - DOI
-
-
See, for example:
- Reisner E. Powell D. J. Cavazza C. Fontecilla-Camps J. C. Armstrong F. A. J. Am. Chem. Soc. 2009;131:18457–18466. doi: 10.1021/ja907923r. - DOI - PubMed
- Brown K. A. S. Dayal S. Ai X. Rumbles G. King P. W. J. Am. Chem. Soc. 2010;132:9672–9680. doi: 10.1021/ja101031r. - DOI - PubMed
- Brown K. A. Wilker B. Boehm M. Dukovic G. King P. W. J. Am. Chem. Soc. 2012;134:5627–5636. doi: 10.1021/ja2116348. - DOI - PubMed
- Honda Y. Hagiwara H. Ida S. Ishihara T. Angew. Chem. Int. Ed. 2016;55:8045–8048. doi: 10.1002/anie.201600177. - DOI - PubMed
-
-
-
Revisions:
- Schilter L. Camara J. M. Huynh M. T. Hammes-Schiffer S. Rauchfuss T. B. Chem. Rev. 2016;116:8693–8749. doi: 10.1021/acs.chemrev.6b00180. - DOI - PMC - PubMed
- Li Y. Rauchfuss T. B. Chem. Rev. 2016;116:7043–7077. doi: 10.1021/acs.chemrev.5b00669. - DOI - PMC - PubMed
- Gloaguen F. Inorg. Chem. 2016;55:390–398. doi: 10.1021/acs.inorgchem.5b02245. - DOI - PubMed
-
Representative examples:
- Li H. Rauchfuss T. B. J. Am. Chem. Soc. 2002;124:726–727. doi: 10.1021/ja016964n. - DOI - PubMed
- Na Y. Pan J. Wang M. Sun L. Inorg. Chem. 2007;46:3813–3815. doi: 10.1021/ic070234k. - DOI - PubMed
- Gao S. Fan J. Sun S. Peng X. Zhao X. Hou J. Dalton Trans. 2008:2128–2135. doi: 10.1039/B717497G. - DOI - PubMed
- Li P. Wang M. Chen L. Liu J. Zhao Z. Sun L. Dalton Trans. 2009:1919–1926. doi: 10.1039/B814336F. - DOI - PubMed
- Apfel U.-P. Troegel D. Halpin Y. Tschierlei S. Uhlemann U. Görls H. Schmitt M. Popp J. Dunne P. Venkatesan M. Coey M. Rudolph M. Vos J. G. Tacke R. Weigand W. Inorg. Chem. 2010;49:10117–10132. doi: 10.1021/ic101399k. - DOI - PubMed
- Camara J. M. Rauchfuss T. B. Nat. Chem. 2012;4:26–30. doi: 10.1038/nchem.1180. - DOI - PMC - PubMed
- Zheng D. Wang M. Chen L. Wang N. Sun L. Inorg. Chem. 2014;53:1555–1561. doi: 10.1021/ic4025519. - DOI - PubMed
- Yu T. Zeng Y. Chen J. Li Y.-Y. Yang G. Li Y. Angew. Chem. Int. Ed. 2013;52:5631–5635. doi: 10.1002/anie.201301289. - DOI - PubMed
-
-
-
An alternative route to incorporate moieties II and III in substrates incompatible with the standard synthetic approaches to these compounds is the use of Fe2[(μ-SCH2)2(NC6H4N3)](CO)6 reagents through a Cu-catalyzed alkyne-azide cycloaddition. See,
- Merinero A. D. Collado A. Casarrubios L. Gómez-Gallego M. Ramírez de Arellano C. Caballero A. Zapata F. Sierra M. A. Inorg. Chem. 2019;58:16267–16278. doi: 10.1021/acs.inorgchem.9b02813. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Research Materials

