Synthesis and photoluminescence properties of hybrid 1D core-shell structured nanocomposites based on ZnO/polydopamine
- PMID: 35518237
- PMCID: PMC9056168
- DOI: 10.1039/d0ra04829a
Synthesis and photoluminescence properties of hybrid 1D core-shell structured nanocomposites based on ZnO/polydopamine
Abstract
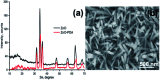
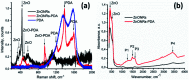
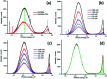
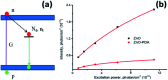
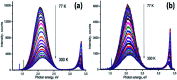
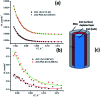
In the present work, we report on the modelling of processes at the zinc oxide and polydopamine (ZnO/PDA) interface. The PDA layer was deposited onto ZnO nanorods (NRs) via chemical bath deposition. The defect concentrations in ZnO before and after PDA deposition were calculated and analysed. The ZnONRs/PDA core-shell nanostructures were studied by transmission electron microscopy (TEM), X-ray diffraction (XRD), Raman and Fourier-transform infrared (FTIR) spectroscopy, photoluminescence (PL) measurements, and diffuse reflectance spectroscopy. The TEM and electron energy loss spectroscopy (EELS) measurements confirmed the conformal coating of PDA, while the PL emission from ZnO and ZnONRs/PDA samples showed a reduction of intensity after the PDA deposition. The decrease of defect concentration participating in PL and quantum efficiency explains the PL reduction. Finally, the observed decrease of activation energies and a shift of the PL peaks are attributed to the formation of an additional local electrical field between the PDA and ZnO nanostructures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
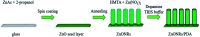

References
-
- Viter R. Savchuk M. Starodub N. Balevicius Z. Tumenas S. Ramanaviciene A. Jevdokimovs D. Erts D. Iatsunskyi I. Ramanavicius A. Sens. Actuators, B. 2019;285:601–606. doi: 10.1016/j.snb.2019.01.054. - DOI
-
- Graniel O. Fedorenko V. Viter R. Iatsunskyi I. Nowaczyk G. Weber M. Załęski K. Jurga S. Smyntyna V. Miele P. J. Mater. Sci. Eng. B. 2018;236:139–146. doi: 10.1016/j.mseb.2018.11.007. - DOI
-
- Man M. T. Kim J.-H. Jeong M. S. Do A.-T. T. Lee H. S. J. Lumin. 2017;185:17–22. doi: 10.1016/j.jlumin.2016.12.046. - DOI
LinkOut - more resources
Full Text Sources

