Open source fraction collector/MALDI spotter for proteomics
- PMID: 35518277
- PMCID: PMC9062586
- DOI: 10.1016/j.ohx.2022.e00305
Open source fraction collector/MALDI spotter for proteomics
Abstract
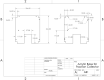
We describe a complete open-source hardware/software solution for high performance thermostatted peptide fraction collection to support mass spectrometry experiments with complex proteomes. The instrument is easy to assemble using parts readily available through retail channels at a fraction of the cost compared to typical commercial systems. Control software is written in Python allowing for rapid customization. We demonstrate several useful applications, including the automated deposition of LC separated peptides for matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) as well as collection and concatenation of peptide fractions from nanoflow HPLC separations.
Keywords: Fraction collection; HPLC, high pressure liquid chromatography; LC, liquid chromatography; LC-MALDI; LC-MS, Liquid Chromatography/Mass spectrometry; MALDI-MS, matrix-assisted laser desorption ionization mass spectrometry; MS, mass spectrometer/mass spectrometry; OEM, Original Equipment Manufacturer; Open source; Proteomics; Python.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
























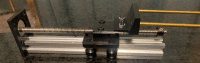














































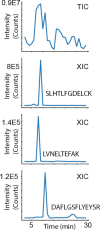
Similar articles
-
LC-MALDI-TOF/TOF for shotgun proteomics.Methods Mol Biol. 2014;1156:27-38. doi: 10.1007/978-1-4939-0685-7_2. Methods Mol Biol. 2014. PMID: 24791979
-
Coupling of nanoflow liquid chromatography to matrix-assisted laser desorption/ionization mass spectrometry: real-time liquid chromatography run mapping on a MALDI plate.Rapid Commun Mass Spectrom. 2004;18(24):3008-14. doi: 10.1002/rcm.1723. Rapid Commun Mass Spectrom. 2004. PMID: 15536631
-
Chemically-assisted fragmentation combined with multi-dimensional liquid chromatography and matrix-assisted laser desorption/ionization post source decay, matrix-assisted laser desorption/ionization tandem time-of flight or matrix-assisted laser desorption/ionization tandem mass spectrometry for improved sequencing of tryptic peptides.Eur J Mass Spectrom (Chichester). 2005;11(2):169-79. doi: 10.1255/ejms.734. Eur J Mass Spectrom (Chichester). 2005. PMID: 16046801
-
"Polymeromics": Mass spectrometry based strategies in polymer science toward complete sequencing approaches: a review.Anal Chim Acta. 2014 Jan 15;808:56-69. doi: 10.1016/j.aca.2013.10.027. Epub 2013 Oct 21. Anal Chim Acta. 2014. PMID: 24370093 Review.
-
Mass spectrometry-based microbiological testing for blood stream infection.Clin Proteomics. 2020 May 13;17:14. doi: 10.1186/s12014-020-09278-7. eCollection 2020. Clin Proteomics. 2020. PMID: 32435163 Free PMC article. Review.
Cited by
-
A methyltransferase-independent role for METTL1 in tRNA aminoacylation and oncogenic transformation.Mol Cell. 2025 Mar 6;85(5):948-961.e11. doi: 10.1016/j.molcel.2025.01.003. Epub 2025 Jan 31. Mol Cell. 2025. PMID: 39892392
-
Customizable large-scale HPLC fraction collection using low-cost 3D printing.HardwareX. 2024 Dec 10;21:e00612. doi: 10.1016/j.ohx.2024.e00612. eCollection 2025 Mar. HardwareX. 2024. PMID: 39811538 Free PMC article.
-
Impact of BRCA mutations, age, surgical indication, and hormone status on the molecular phenotype of the human Fallopian tube.Nat Commun. 2025 Mar 26;16(1):2981. doi: 10.1038/s41467-025-58145-2. Nat Commun. 2025. PMID: 40140386 Free PMC article.
References
-
- Washburn M.P., Wolters D., Yates J.R. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 2001;19(3):242–247. - PubMed
-
- Zhou F., Sikorski T.W., Ficarro S.B., Webber J.T., Marto J.A. Online nanoflow reversed phase-strong anion exchange-reversed phase liquid chromatography-tandem mass spectrometry platform for efficient and in-depth proteome sequence analysis of complex organisms. Anal. Chem. 2011;83(18):6996–7005. - PMC - PubMed
-
- Brown F.C., Still E., Koche R.P., Yim C.Y., Takao S., Cifani P., Reed C., Gunasekera S., Ficarro S.B., Romanienko P., Mark W., McCarthy C., de Stanchina E., Gonen M., Seshan V., Bhola P., O'Donnell C., Spitzer B., Stutzke C., Lavallee V.P., Hebert J., Krivtsov A.V., Melnick A., Paietta E.M., Tallman M.S., Letai A., Sauvageau G., Pouliot G., Levine R., Marto J.A., Armstrong S.A., Kentsis A. MEF2C phosphorylation is required for chemotherapy resistance in acute myeloid leukemia. Cancer Discov. 2018;8:478–497. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

