Fluoxetine scaffold to design tandem molecular antioxidants and green catalysts
- PMID: 35518299
- PMCID: PMC9053872
- DOI: 10.1039/d0ra03509b
Fluoxetine scaffold to design tandem molecular antioxidants and green catalysts
Abstract
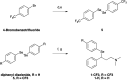
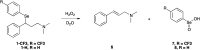
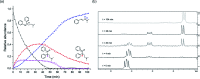
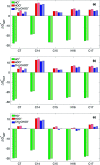
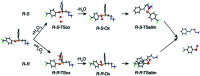
Fluoxetine finds application in the treatment of depression and mood disorders. This selective serotonin-reuptake inhibitor (SSRI) also contrasts oxidative stress by direct ROS scavenging, modulation of the endogenous antioxidant defense system, and/or enhancement of the serotonin antioxidant capacity. We synthesised some fluoxetine analogues incorporating a selenium nucleus, thus expanding its antioxidant potential by enabling a hydroperoxides-inactivating, glutathione peroxidase (GPx)-like activity. Radical scavenging and peroxidatic activity were combined in a water-soluble, drug-like, tandem antioxidant molecule. Selenofluoxetine derivatives were reacted with H2O2 in water, and the mechanistic details of the reaction were unravelled combining nuclear magnetic resonance (NMR), electrospray ionisation-mass spectrometry (ESI-MS) and quantum chemistry calculations. The observed oxidation-elimination process led to the formation of seleninic acid and cinnamylamine in a trans-selective manner. This mechanism is likely to be extended to other substrates for the preparation of unsaturated cinnamylamines.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
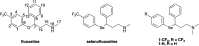
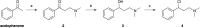
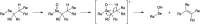
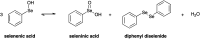
References
LinkOut - more resources
Full Text Sources

