Cascade annulation reaction (CAR): highly diastereoselective synthesis of pyranopyrazole scaffolds
- PMID: 35518344
- PMCID: PMC9053953
- DOI: 10.1039/d0ra03400b
Cascade annulation reaction (CAR): highly diastereoselective synthesis of pyranopyrazole scaffolds
Abstract
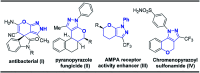
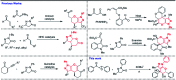
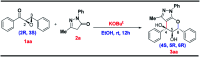
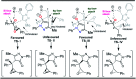
An unprecedented domino protocol for the novel synthesis of highly diverse and functionalized tetrahydro pyranopyrazole scaffolds using chalcone epoxide has been reported for the first time. This synthetic protocol generates three consecutive stereogenic centres in a highly diastereoselective manner with the formation of vicinal diol and a quaternary carbon centre. A wide range of substrates were utilized for the scope of this methodology and provided very good yields of pyranopyrazoles. The pyranopyrazoles were also transformed into densely functionalized tetrasubstituted olefins.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Zheng Y. Cui L. Wang Y. Zhou Z. J. Org. Chem. 2016;81:4340. doi: 10.1021/acs.joc.6b00196. - DOI - PubMed
- Ansari A. Ali A. Asif M. Shamsuzzaman New J. Chem. 2017;41:16. doi: 10.1039/C6NJ03181A. - DOI
- Li W. Ruzi R. Ablajan K. Tetrahedron. 2017;73:164. doi: 10.1016/j.tet.2016.11.067. - DOI
- Faria J. V. Vegi P. F. Miguita A. G. C. dos Santos M. S. Boechat N. Bernardino A. M. R. Bioorg. Med. Chem. 2017;25:5891. doi: 10.1016/j.bmc.2017.09.035. - DOI - PubMed
- Kücųükgüzel S. G. Şenkardes S. Eur. J. Med. Chem. 2015;97:786. doi: 10.1016/j.ejmech.2014.11.059. - DOI - PubMed
-
- Penning T. D. Talley J. J. Bertenshaw S. R. Carter J. S. Collins P. W. Docter S. Graneto M. J. Lee L. F. Malecha J. W. Miyashiro J. M. Rogers R. S. Rogier D. J. Yu S. S. Anderson G. D. Burton E. G. Cogburn J. N. Gregory S. A. Koboldt C. M. Perkins W. E. Seibert K. Veenhuizen A. W. Zhang Y. Y. Isakson P. C. J. Med. Chem. 1997;40:1347. doi: 10.1021/jm960803q. - DOI - PubMed
- Terrett N. K. Bell A. S. Brown D. Ellis P. Bioorg. Med. Chem. Lett. 1996;6:1819. doi: 10.1016/0960-894X(96)00323-X. - DOI
- Despotopoulou C. Klier L. Knochel P. Org. Lett. 2009;11:3326. doi: 10.1021/ol901208d. - DOI - PubMed
-
- Kurosawa E. Fukuzawa A. Irie T. Tetrahedron Lett. 1973;14:4135. doi: 10.1016/S0040-4039(01)87131-8. - DOI
- Suzuki M. Kurata K. Kurosawa E. Bull. Chem. Soc. Jpn. 1986;59:2953. doi: 10.1246/bcsj.59.2953. - DOI
- Fukuzawa A. Masamune T. Tetrahedron Lett. 1981;22:4081. doi: 10.1016/S0040-4039(01)82070-0. - DOI
- Audouze K. Nielsen E. O. Peters D. J. Med. Chem. 2004;47:3089. doi: 10.1021/jm031111m. - DOI - PubMed
- Penga G. P. Tiana G. Huanga X. F. Lou F. C. Phytochemistry. 2003;63:877. doi: 10.1016/S0031-9422(03)00222-X. - DOI - PubMed
- Rodriguez A. D. Cobar O. M. Padilla O. L. J. Nat. Prod. 1997;60:915. doi: 10.1021/np970215v. - DOI - PubMed
-
- Shaabani A. Sarvary A. Keshipour S. Tetrahedron. 2009;65:3492. doi: 10.1016/j.tet.2009.02.035. - DOI
- Aleem M. Remaily A. Tetrahedron. 2014;70:2971. doi: 10.1016/j.tet.2014.03.024. - DOI
- Paul S. Pradhan K. Das A. R. Tetrahedron. 2014;70:6088. doi: 10.1016/j.tet.2014.02.077. - DOI
- Xie J. Sha F. Wu X. Tetrahedron. 2016;72:4047. doi: 10.1016/j.tet.2016.05.033. - DOI
- Seydimemet M. Ablajan K. Obul M. Tetrahedron. 2016;72:7599. doi: 10.1016/j.tet.2016.10.016. - DOI
LinkOut - more resources
Full Text Sources

