Enzymatic preparation of glycerophosphatilcholine catalyzed by combinational phospholipases: a comparative study of concerted versus stepwise catalysis
- PMID: 35518402
- PMCID: PMC9057254
- DOI: 10.1039/d0ra07012b
Enzymatic preparation of glycerophosphatilcholine catalyzed by combinational phospholipases: a comparative study of concerted versus stepwise catalysis
Abstract
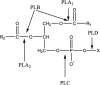
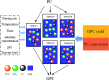
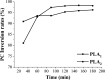
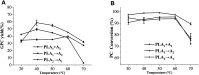
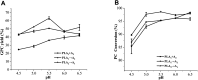
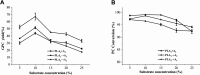
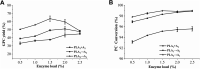
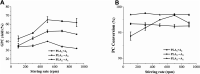
Glycerophosphatilcholine (GPC) is widely applied in medical, pharmaceutical, food and cosmetic industries. Due to the lack of natural resources, enzymatic preparation of GPC has been explored in recent years. This study aimed to investigate and compare the effects of different addition methods of combinational phospholipases (PLA1 and PLA2) and various process parameters (time, temperature, pH, substrate concentrate, enzyme load, and stirring rate) on the preparation of GPC. The results showed that compared with concerted catalysis, the catalytic efficiency of adding PLA2 and then PLA1 (PLA2 → A1) was higher, whereas that of adding PLA1 and then PLA2 was lower. The main reason might be that the method of PLA2 → A1 could reduce acyl migration and the competition between PLA1 and PLA2, which was beneficial to improve the GPC yield and shorten the reaction time. This paper could provide a novel approach for the future preparation of GPC catalyzed by combinational phospholipases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

Similar articles
-
Two-stage Enzymatic Hydrolysis of Soybean Concentrated Phospholipid to Prepare Glycerylphosphorylcholine: Optimized by Response Surface Methodology.J Oleo Sci. 2021 Feb 1;70(2):237-245. doi: 10.5650/jos.ess20261. Epub 2021 Jan 15. J Oleo Sci. 2021. PMID: 33456010
-
A novel fluorogenic substrate for the measurement of endothelial lipase activity.J Lipid Res. 2011 Feb;52(2):374-82. doi: 10.1194/jlr.D007971. J Lipid Res. 2011. PMID: 21062953 Free PMC article.
-
Activity of phospholipases A and lysophospholipase in turkey semen and oviducal fluid.Poult Sci. 2004 Aug;83(8):1385-93. doi: 10.1093/ps/83.8.1385. Poult Sci. 2004. PMID: 15339015
-
Current Knowledge on Mammalian Phospholipase A1, Brief History, Structures, Biochemical and Pathophysiological Roles.Molecules. 2022 Apr 12;27(8):2487. doi: 10.3390/molecules27082487. Molecules. 2022. PMID: 35458682 Free PMC article. Review.
-
Phospholipases in biology and medicine.Clin Biochem. 1990 Oct;23(5):349-70. doi: 10.1016/0009-9120(90)90051-u. Clin Biochem. 1990. PMID: 2253331 Review.
References
LinkOut - more resources
Full Text Sources

