Circular linkage of intramolecular multi-hydrogen bonding frameworks through nucleophilic substitutions of β-dicarbonyls onto cyanuric chloride and subsequent tautomerisation
- PMID: 35518411
- PMCID: PMC9057380
- DOI: 10.1039/d0ra07677e
Circular linkage of intramolecular multi-hydrogen bonding frameworks through nucleophilic substitutions of β-dicarbonyls onto cyanuric chloride and subsequent tautomerisation
Abstract
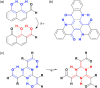
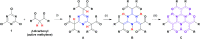
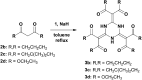
Nucleophilic substitution reactions of cyanuric chloride with a series of β-dicarbonyls give triply β-dicarbonyl-embedded 1,3,5-triazines. Their subsequent but spontaneous tautomeric transformation leads to circularly linked, intramolecular, multi-hydrogen bonding networks. Their structural elucidation by X-ray crystallography showed elongated double bonds and shortened single bonds. This is likely due to a resonance hybrid formed via tautomerisation and simultaneous proton transfer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Hydration effect on proton transfer in melamine-cyanuric acid complex.J Mol Model. 2016 Jul;22(7):169. doi: 10.1007/s00894-016-3038-5. Epub 2016 Jun 28. J Mol Model. 2016. PMID: 27351422
-
The physicochemical essence of the purine·pyrimidine transition mismatches with Watson-Crick geometry in DNA: A·C* versa A*·C. A QM and QTAIM atomistic understanding.J Biomol Struct Dyn. 2015;33(1):28-55. doi: 10.1080/07391102.2013.852133. Epub 2013 Nov 21. J Biomol Struct Dyn. 2015. PMID: 24261751
-
The nature of solid-state N-H triplebondO/O-H triplebond N tautomeric competition in resonant systems. Intramolecular proton transfer in low-barrier hydrogen bonds formed by the triplebond O=C-C=N-NH triple bond --> <-- triplebond HO-C=C-N=N triplebond Ketohydrazone-Azoenol system. A variable-temperature X-ray crystallographic and DFT computational study.J Am Chem Soc. 2002 Nov 13;124(45):13554-67. doi: 10.1021/ja020589x. J Am Chem Soc. 2002. PMID: 12418911
-
Proton transfer reactions and hydrogen-bond networks in protein environments.J R Soc Interface. 2013 Nov 27;11(91):20130518. doi: 10.1098/rsif.2013.0518. Print 2014 Feb 6. J R Soc Interface. 2013. PMID: 24284891 Free PMC article. Review.
-
Ancient Evolution and Recent Evolution Converge for the Biodegradation of Cyanuric Acid and Related Triazines.Appl Environ Microbiol. 2016 Jan 4;82(6):1638-1645. doi: 10.1128/AEM.03594-15. Appl Environ Microbiol. 2016. PMID: 26729715 Free PMC article. Review.
References
-
- Kuhn B. Mohr P. Stahl M. J. Med. Chem. 2010;53:2601. doi: 10.1021/jm100087s. - DOI - PubMed
- Scheiner S. Molecules. 2017;22:1521. doi: 10.3390/molecules22091521. - DOI
- Sánchez G. Molecules. 2019;24:2858. doi: 10.3390/molecules24162858. - DOI
- Caron G. Kihlberg J. Ermondi G. Med. Res. Rev. 2019;39:1707–1729. doi: 10.1002/med.21562. - DOI - PubMed
-
. and the references therein
-
- Tanaka Y. Shin J.-Y. Osuka A. Eur. J. Org. Chem. 2008:1341–1349. doi: 10.1002/ejoc.200701132. - DOI
- Xie Y.-S. Yamaguchi K. Toganoh M. Uno H. Suzuki M. Mori S. Saito S. Osuka A. Furuta H. Angew. Chem., Int. Ed. 2009;48:5496–5499. doi: 10.1002/anie.200900596. - DOI - PubMed
- Soya T. Kim W. Kim D. Osuka A. Chem.–Eur. J. 2015;21:8341–8346. doi: 10.1002/chem.201500650. - DOI - PubMed
- Li C. Zhang K. Ishida M. Li Q. Shimomura K. Baryshnikov G. Li X. Savage M. Wu X.-Y. Yang S. Furuta H. Xie Y. Chem. Sci. 2020;11:2790–2795. doi: 10.1039/C9SC06197E. - DOI - PMC - PubMed
-
- Woolfe G. J. Melzig M. Schneider S. Dorr F. C. Chem. Phys. 1983;77:213–221. doi: 10.1016/0301-0104(83)85078-2. - DOI
- Tobita S. Yamamoto M. Kurahayashi N. Tsukagoshi R. Nakamura Y. Shizuka H. J. Phys. Chem. A. 1998;102:5206–5214. doi: 10.1021/jp981368+. - DOI
- Padalkar V. S. Seki S. Chem. Soc. Rev. 2016;45:169–202. doi: 10.1039/C5CS00543D. - DOI - PubMed
-
- Peng C.-Y. Shen J.-Y. Chen Y.-T. Wu P.-J. Hung W.-Y. Hu W.-P. Chou P.-T. J. Am. Chem. Soc. 2015;137:14349–14357. doi: 10.1021/jacs.5b08562. - DOI - PubMed
- Chen Y.-T. Wu P.-J. Peng C.-Y. Shen J.-Y. Tsai C.-C. Hu W.-P. Chou P.-T. Phys. Chem. Chem. Phys. 2017;19:28641–28646. doi: 10.1039/C7CP05002J. - DOI - PubMed
- Mamada M. Inada K. Komino T. Potscavage Jr W. J. Nakanotani H. Adachi C. ACS Cent. Sci. 2017;3:769–777. doi: 10.1021/acscentsci.7b00183. - DOI - PMC - PubMed
-
- Cha W. Y. Soya T. Tanaka T. Mori H. Hong Y. Lee S. Park K. H. Osuka A. Kim D. Chem. Commun. 2016;52:6076–6078. doi: 10.1039/C6CC02051H. - DOI - PubMed
- Soya T. Mori H. Hong Y. Koo Y. H. Kim D. Osuka A. Angew. Chem., Int. Ed. 2017;56:3232–3236. doi: 10.1002/anie.201700607. - DOI - PubMed
- Umetani M. Kim J. Tanaka T. Kim D. Osuka A. Chem. Commun. 2019;55:10547–10550. doi: 10.1039/C9CC05580K. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

