NH2-MIL-88B (Fe α In1- α ) mixed-MOFs designed for enhancing photocatalytic Cr(vi) reduction and tetracycline elimination
- PMID: 35518441
- PMCID: PMC9057341
- DOI: 10.1039/d0ra07487j
NH2-MIL-88B (Fe α In1- α ) mixed-MOFs designed for enhancing photocatalytic Cr(vi) reduction and tetracycline elimination
Abstract
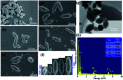
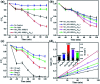
Aiming at solving the issue of wastewater purification, this work synthesized NH2-MIL-88B (Fe α In1-α ) photocatalysts by a simple one-pot method, which was employed for photocatalytic reduction of Cr(vi) and oxidation of TC-HCl. Compared with traditional NH2-MIL-88B (Fe) photocatalysts, NH2-MIL-88B (Fe0.6In0.4) displayed excellent photocatalytic performance; the photocatalytic redox rate for Cr(vi) and TC-HCl reached 86.83% and 72.05%, respectively. The good photocatalytic performance might be attributed to the metal-to-metal charge transition (MMCT) between Fe-O clusters and In-O clusters formed by doping In(iii) into NH2-MIL-88B (Fe), which provides effective active sites for the photocatalytic reduction and oxidation routes. Besides, the synergistic effect of the ligand-to-metal charge transition (LMCT) and MMCT expands the separation and transfer of photogenerated carriers and inhibits the recombination of electron-hole pairs, thus effectively improving the photocatalytic performance. Therefore, this work could provide a new method for the construction of mixed metal MOFs for the photocatalytic degradation of pollutants.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Yi X. H. Wang F. X. Du X. D. Fu H. Wang C. C. Polyhedron. 2018;152:216–224. doi: 10.1016/j.poly.2018.06.041. - DOI
-
- Xie H. Zhang J. Wang D. Liu J. Wang L. Xiao H. Appl. Surf. Sci. 2020;504:144456. doi: 10.1016/j.apsusc.2019.144456. - DOI
-
- Zeng L. Li X. Fan S. Zhang M. Yin Z. Tadé M. Liu S. ACS Appl. Energy Mater. 2018;1:3752–3762. doi: 10.1021/acsaem.8b00524. - DOI
-
- Zeng L. Li X. Fan S. Yin Z. Mu J. Qin M. Chen A. J. Power Sources. 2020;478:228755. doi: 10.1016/j.jpowsour.2020.228755. - DOI
LinkOut - more resources
Full Text Sources

