Enhanced oxygen evolution reaction on amine functionalized graphene oxide in alkaline medium
- PMID: 35518457
- PMCID: PMC9060968
- DOI: 10.1039/c8ra10286d
Enhanced oxygen evolution reaction on amine functionalized graphene oxide in alkaline medium
Abstract
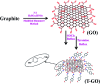
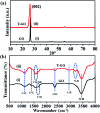
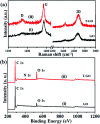
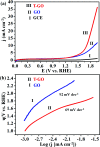
Development of highly efficient oxygen evolution reaction (OER) electrocatalysts is a critical challenge in the cost-effective generation of clean fuels. Here, a metal-free tyramine functionalized graphene oxide (T-GO) electrocatalyst is proposed to use in alkaline electrolytes for enhanced OER. Moreover, the T-GO and GO nanomaterials are well characterized by SEM, XRD, FTIR, XPS and Raman spectroscopy. T-GO exhibits an electrocatalytic OER with a current density of 2 mA cm-2 at a low onset potential of ∼1.39 V and a small Tafel slope of about 69 mV dec-1 and GO exhibits an onset potential of 1.51 V and Tafel slope of about 92 mV dec-1. Additionally, the current stability and RRDE based diffusion controlled response of the T-GO electrocatalyst are outstanding compared to GO. This study establishes metal free T-GO as an efficient electrocatalyst for the OER and used for cathodic production of hydrogen as a counter reaction in the field of water splitting.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Enhanced Overall Water-Splitting Performance: Oleylamine-Functionalized GO/Cu2ZnSnS4 Composite as a Nobel Metal-Free and NonPrecious Electrocatalyst.ACS Omega. 2019 Nov 5;4(21):18969-18977. doi: 10.1021/acsomega.9b01680. eCollection 2019 Nov 19. ACS Omega. 2019. PMID: 31763518 Free PMC article.
-
Hierarchical Fe-doped Ni3Se4 ultrathin nanosheets as an efficient electrocatalyst for oxygen evolution reaction.Nanoscale. 2018 Mar 15;10(11):5163-5170. doi: 10.1039/c8nr00426a. Nanoscale. 2018. PMID: 29492488
-
NiFe Nanoparticle Nest Supported on Graphene as Electrocatalyst for Highly Efficient Oxygen Evolution Reaction.Small. 2024 Apr;20(15):e2308278. doi: 10.1002/smll.202308278. Epub 2023 Nov 27. Small. 2024. PMID: 38009756
-
Edge-selectively phosphorus-doped few-layer graphene as an efficient metal-free electrocatalyst for the oxygen evolution reaction.Chem Commun (Camb). 2016 Oct 27;52(88):13008-13011. doi: 10.1039/c6cc07217h. Chem Commun (Camb). 2016. PMID: 27748479
-
Engineering Bimetallic NiFe-Based Hydroxides/Selenides Heterostructure Nanosheet Arrays for Highly-Efficient Oxygen Evolution Reaction.Small. 2021 Feb;17(7):e2007334. doi: 10.1002/smll.202007334. Epub 2021 Jan 27. Small. 2021. PMID: 33501753 Review.
Cited by
-
Enhanced Overall Water-Splitting Performance: Oleylamine-Functionalized GO/Cu2ZnSnS4 Composite as a Nobel Metal-Free and NonPrecious Electrocatalyst.ACS Omega. 2019 Nov 5;4(21):18969-18977. doi: 10.1021/acsomega.9b01680. eCollection 2019 Nov 19. ACS Omega. 2019. PMID: 31763518 Free PMC article.
-
Catalytic system having an organotellurium ligand on graphene oxide: immobilization of Pd(0) nanoparticles and application in heterogeneous catalysis of cross-coupling reactions.RSC Adv. 2024 Aug 27;14(37):27092-27109. doi: 10.1039/d4ra03401e. eCollection 2024 Aug 22. RSC Adv. 2024. PMID: 39193294 Free PMC article.
-
Fabrication and Integration of Functionalized N-rGO-Ni/Ag and N-rGO-Ni/Co Nanocomposites as Synergistic Oxygen Electrocatalysts in Fuel Cells.Nanomaterials (Basel). 2022 Feb 9;12(4):585. doi: 10.3390/nano12040585. Nanomaterials (Basel). 2022. PMID: 35214913 Free PMC article.
-
Graphene oxide-based electrochemical activation of ethionamide towards enhanced biological activity.RSC Adv. 2019 Nov 1;9(61):35463-35472. doi: 10.1039/c9ra06681k. eCollection 2019 Oct 31. RSC Adv. 2019. PMID: 35528088 Free PMC article.
References
-
- Jin C. Lu F. Cao X. Yang Z. Yang R. J. Mater. Chem. A. 2013;1:12170–12177. doi: 10.1039/C3TA12118F. - DOI
-
- Digraskar R. V. Sapner V. S. Narwade S. S. Mali S. M. Ghule A. V. Sathe B. R. RSC Adv. 2018;8:20341–20346. doi: 10.1039/C8RA01886C. - DOI - PMC - PubMed
- Zou X. Huang X. Goswami A. Silva R. Sathe B. R. Mikmekov E. Asefa T. Angew. Chem., Int. Ed. 2014;53:4372–4376. doi: 10.1002/anie.201311111. - DOI - PubMed
- Yue X. Huang S. Cai J. Jin Y. Shen P. K. J. Mater. Chem. A. 2017;5:7784–7790. doi: 10.1039/C7TA01957B. - DOI
-
- Jiao L. Zhou Y. Jiang H. L. Chem. Sci. 2016;7:1690–1695. doi: 10.1039/C5SC04425A. - DOI - PMC - PubMed
- Maruthapandian V. Mathankumar M. Saraswathy V. Subramanian B. Muralidharan S. ACS Appl. Mater. Interfaces. 2017;9:13132–13141. doi: 10.1021/acsami.6b16685. - DOI - PubMed
- Tao Z. Wang T. Wang X. Zheng J. Li X. ACS Appl. Mater. Interfaces. 2016;8:35390–35397. doi: 10.1021/acsami.6b13411. - DOI - PubMed
- Zhang Y. Fan X. Jian J. Yu D. Zhang Z. Dai L. Energy Environ. Sci. 2017;10:2312–2317. doi: 10.1039/C7EE01702B. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

