Phospholipase D encapsulated into metal-surfactant nanocapsules for enhancing biocatalysis in a two-phase system
- PMID: 35518461
- PMCID: PMC9060939
- DOI: 10.1039/c8ra09827a
Phospholipase D encapsulated into metal-surfactant nanocapsules for enhancing biocatalysis in a two-phase system
Abstract
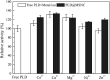
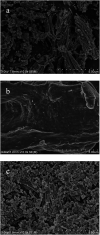
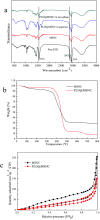
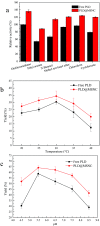
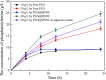
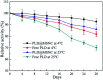
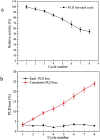
Methods for enhancing enzyme activities in two-phase systems are getting more attention. Phospholipase D (PLD) was successfully encapsulated into metal-surfactant nanocapsules (MSNCs) using a one-pot self-assembly technique in an aqueous solution. The highest yield for the production of high-value phosphatidylserine (PS) from low-value phosphatidylcholine (PC) in the two-phase system was achieved by encapsulating PLD into MSNCs formed from Ca2+ which gave an enzyme activity that was 133.6% of that of free PLD. The PLD@MSNC transformed the two-phase system into an emulsion phase system and improved the organic solvent tolerance, pH and thermal stabilities as well as the storage stability and reusability of the enzyme. Under optimal conditions, PLD@MSNC generated 91.9% PS over 8 h in the two-phase system, while free PLD generated only 77.5%.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Khatoon H. Mansfeld J. Ulbrich-Hofmann R. J. Am. Oil Chem. Soc. 2016;93:365–372. doi: 10.1007/s11746-015-2784-3. - DOI
-
- Scillipoti J. Nioi C. Marty A. Camy S. Condoret J. S. Biochem. Eng. J. 2017;117:162–171. doi: 10.1016/j.bej.2016.10.012. - DOI
-
- Arca-Ramos A. Eibes G. Moreira M. T. Feijoo G. Lema J. M. Process Biochem. 2012;47:1115–1121. doi: 10.1016/j.procbio.2012.04.002. - DOI
-
- Bastida A. Blanco R. M. Zárate S. G. García-Junceda E. Guisán J. M. Biocatal. Biotransform. 2018;36:371–378. doi: 10.1080/10242422.2017.1326484. - DOI
-
- Zhong X. Qian J. Liu M. Ma L. Eng. Life Sci. 2013;13:563–571. doi: 10.1002/elsc.201200170. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

