A colorimetric and ratiometric fluorescent sensor for sequentially detecting Cu2+ and arginine based on a coumarin-rhodamine B derivative and its application for bioimaging
- PMID: 35518477
- PMCID: PMC9060912
- DOI: 10.1039/c8ra09943j
A colorimetric and ratiometric fluorescent sensor for sequentially detecting Cu2+ and arginine based on a coumarin-rhodamine B derivative and its application for bioimaging
Abstract
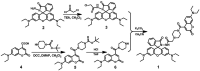
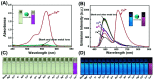
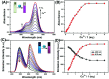
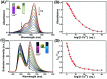
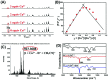
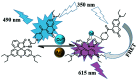
In this work, a colorimetric and ratiometric fluorescent sensor based on a coumarin-rhodamine B hybrid for the sequential recognition of Cu2+ and arginine (Arg) via the FRET mechanism was designed and synthesized. With the addition of Cu2+, the solution displayed a colorimetric change from pale yellow to pink which is discernible by the naked eye. Additionally, the fluorescence intensities of the sensor exhibited ratiometric changes for the detection of Cu2+ at 490 and 615 nm under a single excitation wavelength of 350 nm, which corresponded to the emissions of coumarin and rhodamine B moieties, respectively. The fluorescence color change could be visualized from blue to pink. The limits of detection were determined to be as low as 0.50 and 0.47 μM for UV-vis and fluorescence measurements, respectively. More importantly, the sensor not only can recognize Cu2+ and form a sensor-Cu2+ complex but can also sequentially detect Arg with the resulting complex. The detection limits for Arg were as low as 0.60 μM (UV-vis measurement) and 0.33 μM (fluorescence measurement), respectively. A fluorescence imaging experiment in living cells demonstrated that the fabricated sensor could be utilized in ratiometric fluorescence imaging towards intracellular Cu2+, which is promising for the detection of low-level Cu2+ and Arg with potentially practical significance.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Ryu H. Choi M. G. Cho E. J. Chang S. K. Dyes Pigm. 2018;153:117–124. doi: 10.1016/j.dyepig.2018.02.013. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

