Synthesis and anti-osteoporosis activity of novel Teriparatide glycosylation derivatives
- PMID: 35518599
- PMCID: PMC9055339
- DOI: 10.1039/d0ra05136e
Synthesis and anti-osteoporosis activity of novel Teriparatide glycosylation derivatives
Abstract
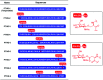
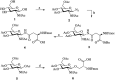
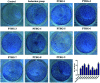
Osteoporosis is a metabolic bone disease that is characterized by low bone mass and micro-architectural deterioration of bones. The mechanism underlying this disease implicates an imbalance between bone resorption and bone remodeling. In 2002, the US Food and Drug Administration (FDA) approved Teriparatide for the treatment of osteoporosis, and so far, this compound is the only permitted osteoanabolic. However, as a structurally flexible linear peptide, this drug may be further optimized. In this study, we develop a series of novel N-acetyl glucosamine glycosylation derivatives of Teriparatide and examine their characteristics. Of the analyzed compounds, PTHG-9 exhibits enhanced helicity, greater protease stability, and increased osteoblast differentiation promoting ability compared with the original Teriparatide. Accordingly, PTHG-9 is suggested as a therapeutic candidate for postmenopausal osteoporosis (PMOP) and other related diseases. The successful development of an enhanced osteoporosis drug proves that the method proposed herein can be used to effectively enhance the chemical and biological properties of linear peptides with various biological functions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
Longitudinal Changes of Circulating miRNAs During Bisphosphonate and Teriparatide Treatment in an Animal Model of Postmenopausal Osteoporosis.J Bone Miner Res. 2021 Jun;36(6):1131-1144. doi: 10.1002/jbmr.4276. Epub 2021 Mar 10. J Bone Miner Res. 2021. PMID: 33598975 Free PMC article.
-
Structure-based development of an osteoprotegerin-like glycopeptide that blocks RANKL/RANK interactions and reduces ovariectomy-induced bone loss in mice.Eur J Med Chem. 2018 Feb 10;145:661-672. doi: 10.1016/j.ejmech.2018.01.022. Epub 2018 Jan 10. Eur J Med Chem. 2018. PMID: 29348072
-
Design, Synthesis, and Anti-Osteoporotic Characterization of Arginine N-Glycosylated Teriparatide Analogs via the Silver-catalyzed Solid-Phase Glycosylation Strategy.J Med Chem. 2024 Jan 25;67(2):1360-1369. doi: 10.1021/acs.jmedchem.3c01903. Epub 2024 Jan 9. J Med Chem. 2024. PMID: 38195392
-
Osteoanabolic therapy for osteoporosis in women.Climacteric. 2022 Feb;25(1):60-66. doi: 10.1080/13697137.2021.1953463. Epub 2021 Aug 3. Climacteric. 2022. PMID: 34342243 Review.
-
Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis.Treat Endocrinol. 2002;1(3):175-90. doi: 10.2165/00024677-200201030-00005. Treat Endocrinol. 2002. PMID: 15799210 Review.
References
LinkOut - more resources
Full Text Sources

