Bleomycin modulates amyloid aggregation in β-amyloid and hIAPP
- PMID: 35518630
- PMCID: PMC9055351
- DOI: 10.1039/d0ra04949b
Bleomycin modulates amyloid aggregation in β-amyloid and hIAPP
Abstract
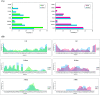
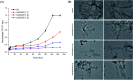
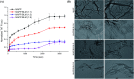
Aberrant misfolding and amyloid aggregation, which result in amyloid fibrils, are frequent and critical pathological incidents in various neurodegenerative disorders. Multiple drugs or inhibitors have been investigated to avert amyloid aggregation in individual peptides, exhibiting sequence-dependent inhibition mechanisms. Establishing or inventing inhibitors capable of preventing amyloid aggregation in a wide variety of amyloid peptides is quite a daunting task. Bleomycin (BLM), a complex glycopeptide, has been widely used as an antibiotic and antitumor drug due to its ability to inhibit DNA metabolism, and as an antineoplastic, especially for solid tumors. In this study, we investigated the dual inhibitory effects of BLM on Aβ aggregation, associated with Alzheimer's disease and hIAPP, which is linked to type 2 diabetes, using both computational and experimental techniques. Combined results from drug repurposing and replica exchange molecular dynamics simulations demonstrate that BLM binds to the β-sheet region considered a hotspot for amyloid fibrils of Aβ and hIAPP. BLM was also found to be involved in β-sheet destabilization and, ultimately, in its reduction. Further, experimental validation through in vitro amyloid aggregation assays was obtained wherein the fibrillar load was decreased for the BLM-treated Aβ and hIAPP peptides in comparison to controls. For the first time, this study shows that BLM is a dual inhibitor of Aβ and hIAPP amyloid aggregation. In the future, the conformational optimization and processing of BLM may help develop various efficient sequence-dependent inhibitors against amyloid aggregation in various amyloid peptides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
No conflict of interest was declared by the authors regarding the content of this research article.
Figures
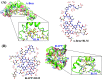

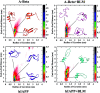
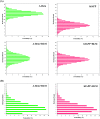
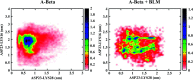

Similar articles
-
Genistein: A Dual Inhibitor of Both Amyloid β and Human Islet Amylin Peptides.ACS Chem Neurosci. 2018 May 16;9(5):1215-1224. doi: 10.1021/acschemneuro.8b00039. Epub 2018 Feb 20. ACS Chem Neurosci. 2018. PMID: 29432676
-
Tanshinones inhibit hIAPP aggregation, disaggregate preformed hIAPP fibrils, and protect cultured cells.J Mater Chem B. 2018 Jan 7;6(1):56-67. doi: 10.1039/c7tb02538f. Epub 2017 Dec 4. J Mater Chem B. 2018. PMID: 32254193
-
The Inhibition Effect of Epigallocatechin-3-Gallate on the Co-Aggregation of Amyloid-β and Human Islet Amyloid Polypeptide Revealed by Replica Exchange Molecular Dynamics Simulations.Int J Mol Sci. 2024 Jan 29;25(3):1636. doi: 10.3390/ijms25031636. Int J Mol Sci. 2024. PMID: 38338914 Free PMC article.
-
Recent computational studies of membrane interaction and disruption of human islet amyloid polypeptide: Monomers, oligomers and protofibrils.Biochim Biophys Acta Biomembr. 2018 Sep;1860(9):1826-1839. doi: 10.1016/j.bbamem.2018.03.006. Epub 2018 Mar 10. Biochim Biophys Acta Biomembr. 2018. PMID: 29530482 Review.
-
Peptide-based amyloid-beta aggregation inhibitors.RSC Med Chem. 2024 Dec 31. doi: 10.1039/d4md00729h. Online ahead of print. RSC Med Chem. 2024. PMID: 39882170 Free PMC article. Review.
Cited by
-
Metabolites from Marine Macroorganisms of the Red Sea Acting as Promoters or Inhibitors of Amylin Aggregation.Biomolecules. 2024 Aug 6;14(8):951. doi: 10.3390/biom14080951. Biomolecules. 2024. PMID: 39199339 Free PMC article.
-
Amyloid Cross-Seeding: Mechanism, Implication, and Inhibition.Molecules. 2022 Mar 8;27(6):1776. doi: 10.3390/molecules27061776. Molecules. 2022. PMID: 35335141 Free PMC article. Review.
References
-
- Dobson C. M. Knowles T. P. Vendruscolo M. The amyloid phenomenon and its significance in biology and medicine. Cold Spring Harbor Perspect. Biol. 2019:a033878.
LinkOut - more resources
Full Text Sources

